PharmaSources/zhulikou431August 15, 2019
Tag: Biopharmaceutical Industry , South Korean , overseas
Hadlima (adalimumab-bwwd) of Samsung Bioepis (a South Korean biopharmaceutical company)/MSD was approved by the U.S. FDA on July 23 as a biosimilar to Humira (generic name: adalimumab) which is a flagship product of AbbVie. It is the third anti-TNF biosimilar of Samsung Bioepis approved in the U.S. and also the fourth biosimilar of the company approved in the U.S.

As the global pharmaceutical industry flourishes, the biopharmaceutical area has also made significant progress. We can see how active the two South Korean pharmaceutical enterprises: Celltrion and Samsung Bioepis have been from the list of biosimilars approved by the U.S. FDA and EU in recent years: among all the 23 biosimilars approved by the U.S. FDA, Celltrion and Samsung Bioepis account for 6 in total.
Table 1 Biosimilars of South Korean Pharmaceutical Enterprises Approved by the FDA
Product name | Company | Approval time | Reference preparation |
Renflexis (infliximab-abda) | Samsung Bioepis/MSD | May 13, 2017 | Remicade |
Truxima | Celltrion | Nov. 28, 2018 | Rituximab |
Herzuma | Celltrion/Teva | Dec. 14, 2018 | Herceptin |
Ontruzant | Samsung Bioepis/MSD | Jan. 21, 2019 | Herceptin |
Eticovo | Samsung Bioepis | Apr. 26, 2019 | Enbrel |
Hadlima (adalimumab-bwwd) | Samsung Bioepis/MSD | July 23, 2019 | Humira |
Let’s first have a rough idea of the basic situation of the South Korean pharmaceutical market (see Fig. 1): the market size of South Korea’s pharmaceutical industry was USD18.7 billion (by 2016), making up 1.8% of approximately USD1.1 trillion of the global pharmaceutical market, 1.2% of South Korean GDP, and 4.3% of South Korean manufacturing GDP. During the same period, South Korean pharmaceutical production was USD16.2 billion with export and import volumes accounting for USD3.1 billion and USD5.6 billion respectively, while South Korean biopharmaceutical production was USD1.73 billion, composing 9.2% of the South Korean pharmaceutical product market. By 2016, there were 737 pharmaceutical enterprises and healthcare supply companies in the country. Of these, 367 manufactured preparations, 204 manufactured APIs, and 379 manufactured quasi-drugs (health products).
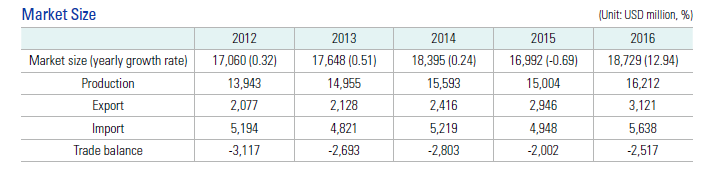
Fig. 1 Market Size of South Korean Pharmaceutical Enterprises
As regulatory and quality management systems for pharmaceutical products have improved with the ongoing efforts of the South Korean pharmaceutical product regulators and pharmaceutical enterprises since 2000, South Korean pharmaceutical products are now receiving great confidence not only from countries in Southeast Asia, Central and South America, but also from those in Europe and the U.S. South Korea MFDS having joined the PIC/s in May, 2014 is an international testament to the quality and management capabilities of South Korean pharmaceutical producers. In addition, the successful gaining of membership to the ICH in Nov. 2016 has won a high international position for South Korea, created a favorable environment for the development of South Korea in global market, and proved that the level of pharmaceutical product regulatory systems in South Korea is on par with that of advanced countries, including the U.S., being one of the countries that is leading in pharmaceutical product regulatory policies.
In terms of new drug R&D investment, South Korea has been increasing the new drug development investment efforts. According to statistics, the average spending on R&D in 2016 by South Korean pharmaceutical producers was 6.0% of sales revenue, and that figure reached 8.9% in listed pharmaceutical producers. In particular, Innovative Pharmaceutical Companies spent 11.7% of revenue, far exceeding the 3.7% recorded in the manufacturing sector. The R&D budget of South Korean pharmaceutical enterprises in 2016 hit USD1.156 billion with an annual average increase of 6.7% over the previous five years.
In terms of new drug R&D, since the successful development of South Korea’s first new drug product "Sunpla Injection (for cancer treatment)" in 1999, over 30 new drugs have been developed in Korea so far. With an average annual roll-out of 1.7 new drugs from 1999 to 2018, South Korea is gradually becoming a global pharmaceutical powerhouse. Furthermore, there are close to 20 drug products that have established a foothold in major global pharmaceutical markets including the U.S. and Europe.
In terms of biopharmaceuticals, South Korea has made amazing achievements: 4 out of the 7 globally commercialized stem cell therapy products were developed in Korea and they include the world’s first stem cell therapy, Hearticellgram-AMI developed by Pharmicell for the treatment of the acute myocardial infarction as an autologous bone marrow mesenchymal stem cell drug. Additionally, a hemophilia treatment NBP601 (AFSTYLA) developed by SK Chemical (with the technical achievement transferred to CSL) became the first new South Korean-developed biologic that obtained a marketing authorization from the U.S. FDA in 2016.
The industry architecture of the Chinese pharmaceutical industry is undergoing subtle changes. The traditional chemical pharmaceutical industry has approached the top in terms of innovation and proportion in the pharmaceutical industry, and the biopharmaceutical industry has increasingly apparent effects on the overall situation of the Chinese pharmaceutical industry as China’s biopharmaceutical industry develops. The attention to and research on the biopharmaceutical industry of South Korean peninsula will be helpful for our expectation and judgement of the industry development for some time to come.
Reference: 2018 DIRECTORY OF KOREAN PHARMACEUTICAL INDUSTRY
Zhulikou431, as a senior engineer, PDA member, ISPE member, ECA member, PQRI member, senior aseptic GMP expert, has deep knowledge in aseptic process development and verification, drug development and registration, CTD document writing and review, regulatory audit, international certification, international registration , quality system construction and maintenance, as well as sterile inspection, environmental monitoring and other fields. In recent years, he has focused on the analysis of trends in the macro pharmaceutical field and the risk management of pharmaceutical enterprise mergers and acquisitions projects.
-----------------------------------------------------------------------
Editor's Note:
If you have any suggestion to the content,
please email: Julia.Zhang@ubmsinoexpo.com


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
Follow Us:




 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025


