PharmaSources/1℃August 14, 2019
Tag: Mitsubishi Tanabe , edaravone , ALS
Edaravone of Mitsubishi Tanabe was approved for marketing in China on July 31, 2019, with the acceptance No.: JXHS1900047 and indication of amyotrophic lateral sclerosis (ALS). The drug was included in the list of 2nd batch of clinically imperative overseas new drugs and was directly filed the marketing application to the NMPA on Apr. 12, 2019
List of 2nd Batch of Clinically Imperative Overseas New Drugs
27 | Radicava (Edaravone) | Mitsubishi Tanabe Pharma Corporation | Japan | June 1, 2015 | Central nervous system | Free radical | Amyotrophic lateral sclerosis | Rare disease drug |
Edaravone is an older drug in my impression. According to my query of the keyword "edaravone" among Chinese-produced pharmaceutical products on the NMPA website, there are 35 records, meaning that there are about 35 specifications of edaravone marketed in China, wherein, edaravone (trade name: Bicun) of Simcere Pharmaceutical has been approved for marketing in China early in 2003.
Can edaravone marketed early in China be used for ALS?
How edaravone of Mitsubishi Tanabe is different?
This article will answer the above questions.
I. Why edaravone of Mitsubishi Tanabe is so excellent?
As mentioned above, there have been dozens of enterprises marketing edaravone in China according to the query on the NMPA website, however, all their indication is ischemic stroke. The marketing application filed by Simcere Pharmaceutical for edaravone dexborneol on Nov. 1, 2018 is still indicated for ischemic stroke, which is only a new compound preparation constituted by edaravone/(+)-2-decanol (4:1), with the safety and effectiveness improved.
Q1: Can Chinese-produced edaravone be used for ALS?
The answer is clear: no!
Edaravone of dozens of Chinese enterprises and hospital supply companies has been approved for stroke, and edaravone compound preparation applied for marketing by Simcere Pharmaceutical is also for stroke, supported by safety and effectiveness data; however, Chinese-produced edaravone has not been verified safety and effectiveness in ALS patients, therefore, the Chinese-produced varieties are different from edaravone of Mitsubishi Tanabe and they must not replace edaravone of Mitsubishi Tanabe to be used for ALS.
Q2: How is Chinese-produced edaravone different from edaravone of Mitsubishi Tanabe?
With the same name "edaravone" but different indications, there must be something different with edaravone of Mitsubishi Tanabe, otherwise, China would not have included it in the list of clinically imperative overseas new drugs to specially accelerate its marketing in China.
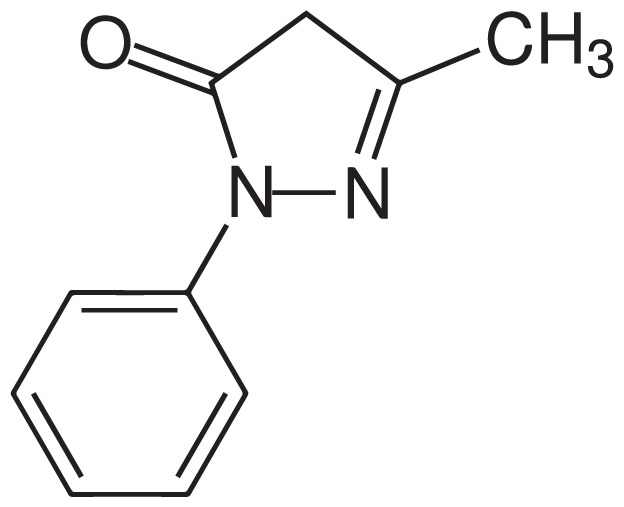
Chemical Structure of Edaravone
Difference 1: Specification
Pharmaceutical product | Classification | Specification |
Edaravone | Chinese-produced variety | 20ml:30mg, injection |
Edaravone | Chinese-produced variety | 5ml:10mg, injection |
Edaravone | Chinese-produced variety | 10ml:15mg, injection |
Edaravone Dexborneol | Chinese-produced variety | 5ml:12.5mg, injection (Edaravone 10mg, (+)-2-decanol 2.5mg) |
Edaravone-ALS | Mitsubishi Tanabe | Injection/infusion bag 100ml:30mg/ 1 infusion bag 100ml:60mg/ 1 infusion bag |
Difference 2: Indication and usage and dosage
Pharmaceutical product | Classification | Indication |
Edaravone | Chinese-produced variety | Stroke, 30 mg/time |
Edaravone | Mitsubishi Tanabe | Stroke, 30 mg/time ALS, 60 mg/time |
As a neuroprotective agent, edaravone has become an oriental miracle drug in China, with annual sales of billions of RMB, however, edaravone produced by Chinese manufacturers has not been conducted any confirmatory clinical trial in ALS indication, which is why they cannot be used for ALS.
II. Milestone events of edaravone of Mitsubishi Tanabe
Edaravone of Mitsubishi Tanabe is discovered in 1984 and developed for stroke over a period of 17 years. Later, it was developed by Mitsubishi Tanabe for ALS, to break the silence of the area for 20 years. The indication has been successively approved in Japan, U.S., and China.
Milestones of edaravone:
1984: Mitsubishi Yuka Pharmaceutical discovered the free radical scavenger: edaravone;
2001: Edaravone (trade name: Radicut), dosage form: 30mg injection, ampoule bottle, was approved for marketing in Japan to treat ischemic stroke;
June 2005: Japan PMDA granted the orphan drug designation to edaravone;
2010: The new dosage form, i.e., 30mg infusion bag, of edaravone was approved in Japan;
May 2015: The FDA granted the orphan drug designation to edaravone;
June 2015: Edaravone (trade name: Radicut) was approved a new indication, i.e., ALS, in Japan;
May 2017: Edaravone (trade name: Radicut) was approved the indication ALS in the U.S.;
Apr. 15, 2019: Mitsubishi Tanabe announced that the marketing application of edaravone (trade name: Radicut) had been accepted by the NMPA, with the indication of ALS;
Jul. 2019: Edaravone of Mitsubishi Tanabe was approved in China for ALS
ALS patent of edaravone
Mitsubishi Tanabe has a patent for this indication in China, with the No.: CN107648227 (A) and application date: Sep. 5, 2012.
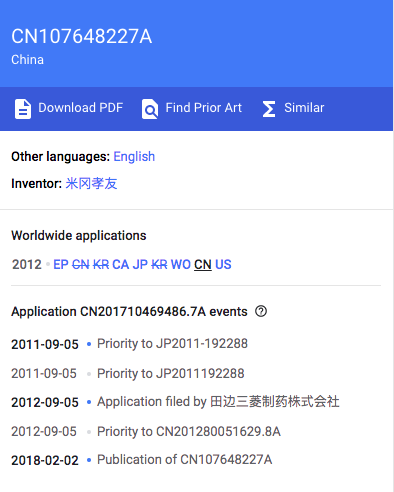
Google patent
Key clinical data NCT01492686 of edaravone
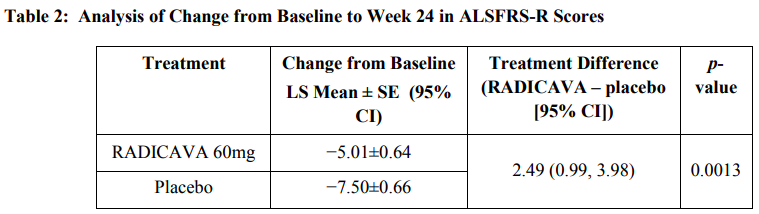
Refer to the prescription insert of edaravone
It took Mitsubishi Tanabe dozens of years to successively develop edaravone for stroke and ALS and launch many clinical trials for ALS; with the clinical data supporting edaravone of Mitsubishi Tanabe (in combination with the standard therapy) for ALS, the drug has successively been approved for the indication in Japan, U.S., and China, to become another ALS drug following riluzole! Therefore, for ALS treatment, edaravone of Mitsubishi Tanabe should be chosen.
-----------------------------------------------------------------------
Editor's Note:
If you have any suggestion to the content,
please email: Julia.Zhang@ubmsinoexpo.com


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
Follow Us:




 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025


