Tim FreemanAugust 12, 2019
Tag: pharmaceutical excipient , Freeman Technology , Tim Freeman , Powder Flow , microcrystalline cellulose
Microcrystalline Cellulose (MCC) has been used as a pharmaceutical excipient for many years, due to its abundance, ease of production and resistance to degradation. As with any excipient, however, variability between batches of supplied material can in turn lead to similar variability in processing, resulting in product that is out of specification, and needs to be reworked or even scrapped. Recent Quality by Design (QbD) initiatives have made it necessary to optimise production processes to ensure the consistency and reliability of final products.
A robust method of quantifying the variations in batches of excipients that contribute to differences in downstream process behaviour enables a design space of acceptable raw material properties to be established. This approach is an essential part of QbD. However, even well-established techniques for material characterisation (such as particle size analysis) do not always provide the required differentiation, as they only evaluate of one physical property of the particles.
Multivariate Analysis of Powder Batches
Three grades of MCC were used as an excipient in the production of pharmaceutical tablets by direct compression. Particle size analysis of the three grades generated almost identical D50 values (100 µm) for each grade, and it was determined that three samples were indistinguishable from each other.
All three samples were further analysed using an FT4 Powder Rheometer®, to evaluate whether differences existed that weren’t identified by the D50 value and consequently whether particle size alone is sufficient as a tool predicting in-process performance.
Test Results
Dynamic Testing: Aeration

Sample C generated a significantly higher Aeration Ratio (AR) than the other samples, demonstrating that its packing structure changes to a greater extent when air is introduced into the sample. This typically indicates a lower degree of cohesive strength between particles. Sample A and Sample B exhibited different responses, with the lower sensitivity to the introduction of air suggesting greater cohesivity compared to Sample C.
Bulk Testing: Permeability
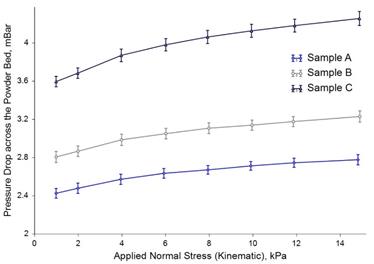
Sample C generated a considerably higher Pressure Drop across the Powder Bed than the other two samples, indicating that it is the least permeable of the three. Low permeability means that any air that becomes entrained in the bulk is less able to escape. Considering the example of a tabletting process, greater air content within the dose would typically lead to capping and lamination as well as weight variation in the final product.
Shear Cell Testing
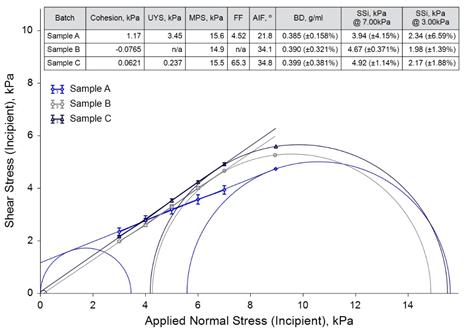
Sample A showed considerably different behaviour to the other two samples, generating the highest Shear Stress values at low normal stress levels, but the lowest values at higher levels. This suggests that the way the powders perform in a given process would be heavily influenced by stress levels they are subjected to, and illustrates the need to characterise powders using process relevant techniques. Considering the results for Sample B, the Yield Locus, generated by a best-fit line through the data points, is so steep that it intercepts the y-axis below the origin. This leads to a negative value for Cohesion, and generates no result for Flow Function (as the minor Mohr circle cannot be constructed in order to produce a value for Unconfined Yield Strength). This illustrates the importance of recording actual measured Shear Stress values alongside parameters derived from Mohr’s circle analysis, as the mathematical model used to obtain these parameters may not always provide data for comparison.
Conclusions
The FT4 has demonstrated clear and repeatable differences between three grades of MCC, suggesting a likely difference in their process performance which is not identified by the single physical property of particle size alone. Of the three samples, Sample A displayed the highest Permeability and the lowest sensitivity to Aeration, as well as being less sensitive to changes in applied stress during Shear Cell tests. This suggests that it is the most efficiently packed of the three samples and is to exhibit more free-flowing behaviour across a range of conditions. Sample C generated the highest Shear Stress values, was most sensitive to Aeration and exhibited the lowest Permeability, suggesting a greater sensitivity to the process conditions than the other samples and illustrating the need to understand the relationship between the process environment and the material properties
Powder flowability is not an inherent material property, but is more about the ability of powder to flow in a desired manner in a specific piece of equipment. Successful processing demands that the powder and the process are well-matched and it is not uncommon for the same powder to perform well in one process but poorly in another. This means that several characterisation methodologies are required, the results from which can be correlated with process ranking to produce a design space of parameters that correspond to acceptable process behaviour. Rather than relying on single number characterisation to describe behaviour across all processes, the FT4’s multivariate approach simulates a range of unit operations, allowing for the direct investigation of a powder’s response to various process and environmental conditions.
Author Biography

Tim Freeman, Managing Director, Freeman Technology
Tim Freeman is Managing Director of powder characterisation company Freeman Technology for whom he has worked since the late 1990s. He was instrumental in the design and continuing development of the FT4 Powder Rheometer® and the Uniaxial Powder Tester. Through his work with various professional bodies, and involvement in industry initiatives, Tim is an established contributor to wider developments in powder processing.
Tim has a degree in Mechatronics from the University of Sussex in the UK. He is a mentor on a number of project groups for the Engineering Research Center for Structured Organic Particulate Systems in the US and a frequent contributor to industry conferences in the area of powder characterisation and processing. A past Chair of the American Association of Pharmaceutical Scientists (AAPS) Process Analytical Technology Focus Group Tim is a member of the Editorial Advisory Board of Pharmaceutical Technology and features on the Industry Expert Panel in European Pharmaceutical Review magazine. Tim is also a committee member of the Particle Technology Special Interest Group at the Institute of Chemical Engineers, Vice-Chair of the D18.24 sub-committee on the Characterisation and Handling of Powders and Bulk Solids at ASTM and a member of the United States Pharmacopeial (USP) General Chapters Physical Analysis Expert Committee (GC-PA EC).
-----------------------------------------------------------------------
Editor's Note:
To become a freelance writer of En-CPhI.CN,
welcome to send your CV and sample works to us,
Email: Julia.Zhang@ubmsinoexpo.com.


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
Follow Us:




 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025


