PharmaSources/MilingJuly 31, 2019
Tag: rare diseases , orphan drugs , Biologics , Small molecule drugs
The new drug Zolgensma of Novartis has lately been approved for marketing by the U.S. FDA to treat pediatric patients with spinal muscular atrophy (SMA). SMA is a rare disease (orphan disease), with the incidence of only 1-2/100,000. It's worth mentioning that as gene therapy, Zolgensma is priced at USD2.125 million/time which is sky-high. This causes people to think about the choice of rare disease drugs: for patients, it is a question as to whether to choose biologics (expensive) or small molecule drugs (cheap); for pharmaceutical practitioners, choosing which drugs for development is also a question for thought.

I. The "orphan drug" legislation behind the expensive prices
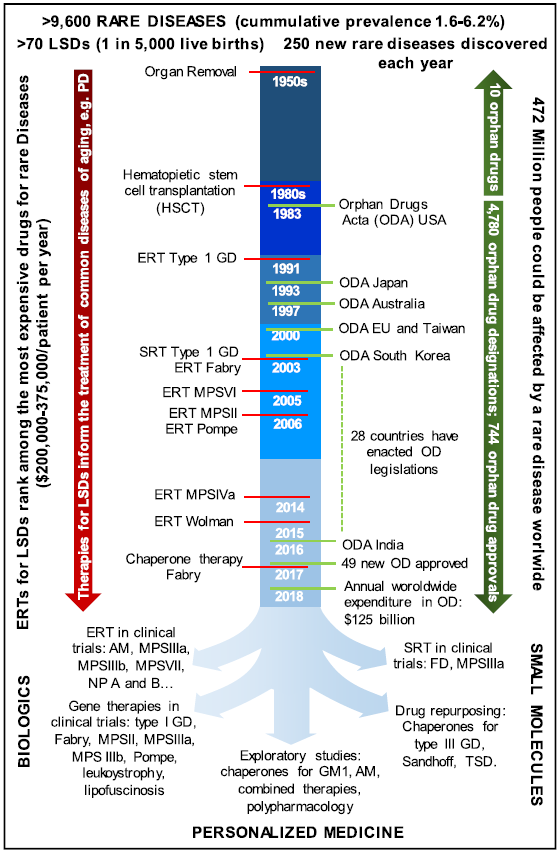
Fig. 1 Chronology of Orphan Disease Legislation and Orphan Drug Development
Development of drugs for rare diseases tends to be relatively slow owing to characteristics such as small patient base and relatively lagging mechanism research thereof; the relatively small market after drug development will often discourage and even kill the enthusiasm of pharmaceutical enterprises for the development of such drugs. As a result, the U.S. took the lead in promulgating the Orphan Drug Act (ODA, Fig. 1) in 1983 and thereafter many countries have legislated for rare diseases in succession. Those acts have undoubtedly promoted orphan drug development to some extent.
However, some ODAs at present have some undeniable drawbacks. Terms such as "independently pricing" and "market exclusivity" will lead to the market monopoly of some orphan drugs and "sky-high prices" unaffordable by most patients, and to some extent promote R&D institutions and enterprises to develop biologics that are generally priced higher and difficult to imitate. According to statistics, biologics have only flourished in recent years, however, rare disease drug approvals have accounted for 36% of the total in recent years, showing a rising trend. According to an insider, expensive drug prices will reduce patients’ access to corresponding drugs and seriously impact the sustainability of the public health system.
II. Biologics or small molecule drugs? Taking lysosomal storage diseases (LSDs) for an instance
Rare diseases are a major public health issue facing each country and are a huge challenge to the medical world.
Market shares of biologics have grown bigger and bigger in recent years owing to the rapid development of biomedicine, which restricts the room of small molecule drugs. Then, regarding biologics and small molecule drugs, which are better rare disease drugs? Whose overall treatment costs are lower? Those are questions for thought.
Fig. 2 Orphan Drug Development
Let us here talk about advantages and disadvantages and development patterns of biologics and small molecule drugs by taking LSDs for instance.
A. Development and advantages and disadvantages of biologics for LSDs
LSDs are monogenetic diseases where the mutation in a gene encoding for a lysosomal protein results in lysosomal failure and the subsequent accumulation of substrates, which ultimately leads to cell dysfunction and cell death. LSDs include Gaucher disease (GD, the LSD with the highest prevalence), Fabry disease, GM1 gangliosidosis (GM1), Pompe disease, α-mannosidosis (AM), Tay–Sachs disease (TSD), Niemann–Pick disease (NP), or the mucopolysaccharidosis (MPS). Most LSDs present as pediatric progressive neurodegenerative diseases.
As a biological therapy, enzyme replacement therapy (ERT) is available for a small proportion of the LSDs (Fig. 1, left side). The ERT developed by Brady and his colleagues for type 1 GD and approved by the FDA in 1991 remains the most successful example in this category.
However, biologics obviously have some disadvantages difficult to overcome, for example, it is often difficult to make clear the distribution of biologics in bodies; biologics are generally macromolecular drugs that are difficult to penetrate the blood-brain barrier (BBB), which restricts their treatment of the LSDs of central nervous system (CNS).
Furthermore, the annual treatment cost for patients using ERT is averagely USD200,000-375,000. How to reduce patients’ treatment cost is an important issue at present with the rapid development of biologics such as gene drugs.
B. Development and advantages and disadvantages of small molecule drugs for LSDs
ERT is currently the mainstream therapy for LSDs, however, such strategy has inherent defects, for example, the recombinant enzymes cannot penetrate the BBB, which limits the application. As such, the substrate reduction therapy (SRT) has been proposed, which aims to reduce glycosphingolipid synthesis to match the degradation activity of lysosome. N-alkylated iminosugars were thought to be applicable for SRT owing to its inhibition of the key enzyme: ceramide glucosyltransferase (CGT) in glycosphingolipid synthesis, and Miglustat (Zaveska) is a representative marketed drug of the N-alkylated iminosugars. Another characteristic of iminosugars is that they, as molecular chaperones, can assist the correct folding and stabilize the conformation of mutant enzymes to rescue their activity. This makes the pharmacological chaperone therapy (PCT) an alternative novel therapeutic strategy for the treatment of LSDs. Guided by this theory, migalastat (Galafold) was approved for marketing in 2017. Iminosugars have common advantages of small molecule drugs, such as high oral availability, strong penetration to the CNS, and evident biological and pharmacological properties.
Summary
The question as to whether to choose biologics or small molecule drugs is like the question as to whether to choose western medicine or traditional Chinese medicine. There has been no great answer to it. Some people say small molecule drugs are often much cheaper for patients, but this is in fact not correct. For example, according to statistics, the separate annual costs of patients with LSDs in using ERT, SRT, and PCT are of the same order of magnitude. Therefore, choosing biologics or small molecules must depend on the development situation of corresponding drugs. We still need to fight the diseases by depending on both biologics and small molecule drugs in the future.
References:
1. Novel Therapies for Orphan Diseases, ACS Med. Chem. Lett. 2019;
2. 氮杂糖应用于溶酶体蓄积症治疗的研究,《中国科学》。
2. Iminosugars for the Treatment of Lysosomal Storage Disorders, Science China
Miling, Master of Pharmacy, majored in biopharmaceutical, has long been engaged in new drug research and development, focusing on the analysis of trends in the domestic and overseas drug market, and is good at the research and development of biological drugs and small molecule drugs.
-----------------------------------------------------------------------
Editor's Note:
If you have any suggestion to the content,
please email: Julia.Zhang@ubmsinoexpo.com


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
Follow Us:




 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025


