PharmaSources/zhixingJuly 30, 2019
Tag: patent , Amgen , Enbrel , Sandoz
Patents are fortresses of new drugs. The patent layout of Enbrel is quite impressive: the span from the first application in 1995 to the patent expiration in 2029 reaches 34 years. Such unrepeatable success has extended the life of Enbrel and stopped the biosimilars. This article gives a brief overview of Enbrel and Amgen’s patent protection therefor.
Enbrel(Etanercept)
As the world’s first fully human tumor necrosis factor (TNF) antagonist, Enbrel (etanercept) was first developed by Immunex and Wyeth-Ayerst and marketed in the U.S. in 1998, with the indication of rheumatoid arthritis (RA), and has now been approved 6 indications by the FDA.
Date | Indication approved by the FDA |
Nov. 2, 1998 | RA |
May 27, 1999 | Juvenile RA |
Jan. 15, 2002 | Psoriatic arthritis |
July 24, 2003 | Ankylosing spondylitis |
Apr. 30, 2004 | Moderate to severe plaque psoriasis |
Nov. 4, 2016 | Pediatric plaque psoriasis |
Amgen has obtained rights to market Enbrel in the U.S. and Canada by acquiring Immunex in 2002, which has soon become a blockbuster drug and main source of sales of Amgen. Sales of Enbrel reached USD5.014 billion in 2018, down by 7.7% compared to 2017.
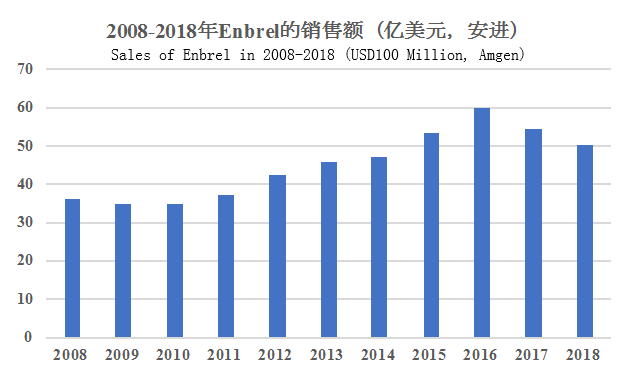
Sales of Enbrel in 2008-2018 (USD100 Million, Amgen)
Sales of Enbrel in the U.S. and Canada from 2008 to 2018 showed the change of rising before declining. The decline of sales in 2017 and 2018 was mainly due to the approvals of other new drugs with same indications, such as Novartis’ new drug Cosentyx (secukinumab) for psoriasis and Pfizer’s new drug Xeljnaz (tofacitinib) for RA, and Remicade, Humira, Stelara, and Otezla also produced pressure on sales of Enbrel. And Enbrel is also faced with threats from biosimilars, in particular, there have been two biosimilars: Benepali and Erelzi marketed in the European market Pfizer is in charge of, and there have also been three biosimilars marketed in China.
Trade name | Pharmaceutical enterprise | Approval date | Approval agency | Status |
Benepali | Samsung Bioepis | Jan.13, 2016 | EMA | Marketed |
Erelzi | Sandoz | June 22, 2017 | EMA | Marketed |
Erelzi | Sandoz | Aug. 30, 2016 | FDA | Patent dance |
Yisaipu | Shanghai Sunshine Guojian | Jan. 27, 2005 | NMPA | Marketed |
Qiangke | Shanghai Celgen | Apr. 11, 2011 | NMPA | Marketed |
Anbainuo | Zhejiang Hisun | Apr. 9, 2015 | NMPA | Marketed |
Amgen’s thorough patent protection for Enbrel
Amgen has been famous for its tight patent protection for new drugs. According to the 2018 annual report of Amgen, there have been four patents to protect the exclusivity of Enbrel in the U.S. market that are still in force, separately, US7915225, US8119604, US8063182, and US8163522.
Patent No. | Content of protection | Expiry date |
US7915225 | Method to treat psoriasis and psoriatic arthritis | Aug. 13. 2019 |
US8119604 | Method to prepare stable liquid preparations and treat diseases | June 8, 2023 |
US8063182 | Fusion protein structure | Nov. 22, 2028 |
US8163522 | Fusion protein DNA coding and the preparation method thereof | Apr. 24, 2029 |
If biosimilars to Enbrel need to be marketed in the U.S., they can bypass the protections to Enbrel from US7915225 and US8119604, namely, being marketed in the lyophilized dosage form or treating other diseases, however, US8063182 and US8163522 have extensive scopes of protection and are difficult to avoid, making them the main obstacle to the marketing of biosimilars to Enbrel.
Roche applied for patents US8063182 and US8163522 in 1995, which have not been timely granted and been kept confidential due to the amendment to the U.S. Patent Act and cannot be published before the granting, therefore, they are called "submarine" and are quite destructive to biosimilars.
Amgen acquired those two "submarine" patents from Roche in 2004 after learning of them, with the grant date separately being 2011 and 2012, and the patent protection periods are up to 17 years from the grant dates, enabling Amgen to extend Enbrel’s exclusivity in the U.S. market to 2029 to become the main weapon against Erelzi of Sandoz in the U.S. market.
Those two "submarine" patents became the obstacle to the marketing of Erelzi in the U.S., therefore, Sandoz requested to the court for the invalidation of US8063182 and US8163522 in 2013 so as to eliminate the obstacle to the marketing of Erelzi, however, the appeal of Sandoz was soon dismissed by the court. Sandoz appealed again in 2014, which was dismissed again by the court.
Sandoz stopped challenging the two "submarine" patents until Erelzi was approved by the FDA in Aug. 2016. Before this, Amgen brought a suit before a court against Sandoz in Feb. 2016, claiming the latter infringing five patents of Enbrel and not complying with the BPCIA, namely, not providing Amgen with the biosimilar marketing application and other information of Erelzi. Amgen and Sandoz reached a consensus in Aug. 2016, according to which Sandoz would not sell Erelzi in the U.S., however, the court still has not given the judgment therefor.
Reference: Amgen/Sandoz website
-----------------------------------------------------------------------
Editor's Note:
If you have any suggestion to the content,
please email: Julia.Zhang@ubmsinoexpo.com


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
Follow Us:




 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025


