PharmaSources/XiaoyaowanJuly 29, 2019
Tag: Cosmetic medicine , Hyaluronic acid , Micro-Plastic
Size of the cosmetic medicine market in China reached RMB122 billion in 2018, making it the second largest cosmetic medicine market in the world. From the perspective of the market segment performance, cosmetic medicine products such as hyaluronic acid led to the rapid growth of the micro-plastic surgery market and are currently used in the most popular cosmetic medicine micro-plastic surgery items.
What is hyaluronic acid?

Hyaluronic acid, also known as hyaluronan, is a sulfur-free straight chain mucopolysaccharide composed of repeat disaccharide units of D-glucuronic acid and N-acetylglucosamine, which widely distributes in human connective tissue, epithelial tissue, and nervous tissue. Hyaluronic acid was separated by Karl Meyer and John Palmer at Columbia University from the vitreous humor of cow eyes in 1934.
Hyaluronic acid is extensively used in the cosmetic medicine industry as a dermal filler, which generally enters the dermis by injection to play the moistening, lubricating, and shaping roles. It can be used as a filler in the micro-plastic surgery field for augmentation rhinoplasty, chin filling, lip augmentation, and wrinkle prevention, etc. or used as a hydro-lifting injection for moisturizing and accounts for a leading share in the cosmetic medicine filler market.
First approved by the FDA in 2003 for skin anti-wrinkle treatment, hyaluronic acid has gradually been used in hydro-lifting injection, etc. for skin moisturizing owing to its moisturizing and moisture-locking functions.
Hyaluronic acid can be divided into macromolecule, middle molecule, and small molecule types depending on the molecular weight. Molecular weight is an important parameter of hyaluronic acid as hyaluronic acids of different molecular weights have different function application fields.
Scope of Application of Different Hyaluronic Acid Molecules | |||
Molecular type | Macromolecule | Middle molecule | Small molecule |
Applicable to sites of | Filling of fine sites and removal of superficial wrinkles, lying silkworm lines, lip wrinkles, neck wrinkles, and nasolabial folds | Filling of soft tissue, removal of deep wrinkles, forehead augmentation, radix nasi augmentation, temple augmentation, filling of lacrimal groove, submalar triangle augmentation, augmentation rhinoplasty, cheek augmentation, lip augmentation, and filling of nose and labial groove | Contour shaping, superciliary arch lifting, radix nasi augmentation, augmentation rhinoplasty, and chin augmentation |
Filling of fine sites and removal of superficial wrinkles, lying silkworm lines, lip wrinkles, neck wrinkles, and nasolabial folds
Filling of soft tissue, removal of deep wrinkles, forehead augmentation, radix nasi augmentation, temple augmentation, filling of lacrimal groove, submalar triangle augmentation, augmentation rhinoplasty, cheek augmentation, lip augmentation, and filling of nose and labial groove
Contour shaping, superciliary arch lifting, radix nasi augmentation, augmentation rhinoplasty, and chin augmentation
Global main origins of hyaluronic acid raw materials
Sales of the hyaluronic acid raw materials reached 4.30 million tons in China in 2018, accounting for 86% of global sales, with the market size of the raw materials reaching RMB3.07 billion. From the perspective of product structure, the Chinese-produced hyaluronic acid raw materials were mainly cosmetic-grade and food-grade raw materials with low added values, and the pharmaceutical-grade raw materials with high added values accounted for a low proportion.
From the perspective of sales, the global top 5 manufacturers of hyaluronic acid raw materials are all Chinese enterprises and healthcare supply companies which, ranked according to the market share, were separately Bloomage Biotech (36%), Focus Chem (12%), Fufeng Biotechnologies (10%), China Eastar Group (8%), and Awa Biopharm (7%).
Hyaluronic acid raw materials and terminal products used in the cosmetic medicine industry are required to be pharmaceutical-grade hyaluronic acid. Raw material manufacturers need to obtain the qualification for producing pharmaceutical-grade hyaluronic acid raw materials, for which there are high requirements on the purity, protein impurity, and heavy metal content, etc. Sales of pharmaceutical-grade raw materials only account for 2.3% of the total sales of hyaluronic acid raw materials in China, which is far lower than the world average level. Chinese manufacturers involved in this area mainly include Bloomage Biotech and Topscience.
Imported products dominating the market of hyaluronic acid as a cosmetic medicine filler in China
The terminal product of pharmaceutical-grade hyaluronic acid in the cosmetic medicine industry is mainly the injection filling beauty products which have high added values and are required to receive the NMPA’s permits for Class 3 medical devices. The market size of the cosmetic medicine hyaluronic acid fillers reached RMB3.7 billion in 2018, with the compound annual growth rate exceeding 17%, to become the main growth driver of the pharmaceutical-grade hyaluronic acid market in China.
Sodium Hyaluronate Products for Injection Marketed in China | |||
Category | Number of varieties | Concentration | Price per milliliter |
Imported products | 12 | 20mg/ml-24mg/ml | High |
Chinese-produced products | 15 | 16mg/ml-20mg/ml | Low |
There is a total of 27 injection products of hyaluronic acid for injection approved for marketing by the NMPA in China, belonging to 14 enterprises including 6 overseas enterprises and 8 Chinese enterprises. Comparatively, imported products have significant brand advantages, most of which are priced higher than Chinese-produced products; the concentration of imported products to China is between 20mg/ml and 24mg/ml, while the concentration of Chinese-produced products is mostly between 16mg/ml and 20mg/ml. Most products can maintain the effects for half a year to one year, which means that they normally need to be used again every half year or every year to maintain the plasticity effects.
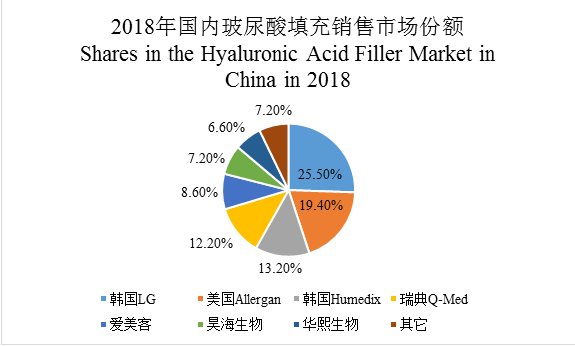
韩国LG | South Korea LG |
美国Allergan | U.S. Allergan |
韩国Humedix | South Korea Humedix |
瑞典Q-Med | Sweden Q-Med |
爱美客 | IMEiK |
昊海生物 | Haohai Biological |
华熙生物 | Bloomage Biotech |
其他 | Others |
From the perspective of sales, the size of the cosmetic medicine hyaluronic acid filler market reached RMB3.7 billion in 2018, wherein, South Korean enterprises and European and American enterprises were separately the top 2 by the market share in China, and their total market shares reached 74.7%, dominating the cosmetic medicine hyaluronic acid filler market in China, with representative enterprises being South Korean enterprises LG (25.50%) and Humedix (13.20%), U.S. Allergan (19.4%), and Sweden Q-Med (12.20%); Chinese enterprises ranked 3rd with total market shares of 23.4%, with the main enterprises including Haohai Biological (18.4%), IMEiK (12.5%), and Bloomage Biotech (11.5%).
The core difference of products of different brands
The half-life of unmodified natural hyaluronic acid is about 1~21 days on sites such as skin, eye, and joint, and such characteristic restricts the application of hyaluronic acid in the cosmetic medicine industry. Changing characteristics of hyaluronic acid through the use of crosslinker and different crosslinking technologies can improve the filling and shaping abilities and durations in vivo of hyaluronic acid to varying degrees.
However, such crosslinking technologies have high barriers; considerations shall especially be given to product safety while improving the hyaluronic acid performance, in particular, safety is far more important than product performance improvement. According to report, a crosslinker that does not bind to hyaluronic acid can bind to DNA when it is free in vivo, producing strong toxicity and carcinogenicity. Therefore, the residue of the crosslinker shall be controlled to the safety range in the actual production process. As such, the crosslinking technology is the core difference of products of different brands and the key for patent layout.
Crosslinkers currently used in the hyaluronic acid filler products certified by the FDA include BDDE (1,4-butanediol diglycidyl ether) and DVS (divinyl sulfone), while, BDDE is recommended and generally used in China’s cosmetic medicine industry for crosslinking.
Xiaoyaowan, a pharmaceutical industry practitioner, a word carrier in the We-media era focusing on changes of the pharma industry.
-----------------------------------------------------------------------
Editor's Note:
If you have any suggestion to the content,
please email: Julia.Zhang@ubmsinoexpo.com


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
Follow Us:




 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025


