Zosano Pharma Corp. has completed manufacturing of site qualification batches at its commercial manufacturing site for Qtrypta, the company’s investigational treatment for migraines in late stage development. These batches are intended to demonstrate the robustness and reproducibility of the manufacturing process which will be included as part of its planned submission of a New Drug Application (NDA) for Qtrypta in 4Q19.
"The completion of these site qualification batches forms the basis of our commercial manufacturing process in our NDA and illustrates the scalability of the process as we head toward commercialization activities for Qtrypta at Thermo Fischer, in North Carolina," said Hayley Lewis, senior vice president, operations at Zosano. "With the completion of our long-term safety study, the transfer of our manufacturing process and the ongoing completion of requisite regulatory documentation and studies, we are on track for NDA submission by year end."
-----------------------------------------------------------------------
Editor's Note:
If you have any suggestion to the content,
please email: Julia.Zhang@ubmsinoexpo.com





 ALL
ALL Pharma in China
Pharma in China Pharma Experts
Pharma Experts Market News
Market News Products Guide
Products Guide Brand Story
Brand Story

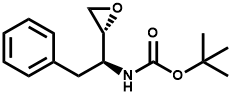
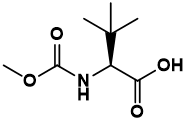


























 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025








