Tim FreemanMay 27, 2019
Tag: OTC , Freeman Technology , wet granules
Tim Freeman, Managing Director at Freeman Technology, explains how comprehensive, process relevant characterisation of wet granules can lead to control of tablet CQAs and assist Design Space definition in the manufacture of an OTC medicine.

The adoption of a Quality by Design (QbD) approach to wet granulation requires manufacturers to fully understand the relationship between process variables, such as powder properties and equipment settings, and Critical Quality Attributes (CQA) of the resulting product. Manufacturers need to develop a Design Space by understanding the impact that variations within the process have on the properties of the resulting granulate and the influence they, in turn, have on final product quality. Furthermore, a robust Design Space allows process variables to be controlled and manipulated to produce quality assured tablets with targeted properties.
Dynamic characterisation for QbD
Variations in material properties, as well as process settings, offer both challenge and opportunity to the manufacturer. Knowledge of how materials, that may themselves vary across batches, behave within a manufacturing process, where conditions may also vary, enable the operator to develop a Design Space within which to operate.
The ability to convert a challenge into an opportunity when working with powders is greatly enhanced if the characterisation techniques employed deliver results that are repeatable, reliable and relate specifically to conditions within the process. Unlike many other established powder flow testing techniques, such as tapped density, angle of repose and shear cells, dynamic test methods simulate typical process conditions and therefore deliver information on materials properties that are more likely to influence final product quality.
Evaluating wet granules
Dynamic testing using a powder rheometer can be used to evaluate the characteristics of wet granules by measuring the resistance that the granulate exerts on a blade, as it traverses through a sample. This resistance is expressed as a “flow energy”, which is calculated from direct measurements of rotational torque and axial force during the blade’s traverse.
Flow energy is influenced by many properties including inter-particular friction and mechanical interlocking, strength of capillary bonds and cohesion between particles. In high shear wet granulation (HSWG), the addition of water and work (shear) results in larger, denser, more adhesive granules which typically generate a higher flow energy as these larger, denser granules are harder to displace using the blade, as well as being less compressible.
Flow energy, often presented as Basic Flowability Energy or BFE, is therefore a function of solid to water ratio, impeller speed and binder temperature, and this robust relationship between flow energy and process settings allows Critical Process Parameters to be identified and a Design Space to be determined. The ability to test in a wet state allows this approach to be applied as early as possible in the manufacturing process.
The following case study shows how a fully functional Design Space, based on dynamic flow properties, can be defined for a wet granulation process enabling CQAs of the final tablet to be targeted.
Case study: Defining wet granulation by granule quality
Two aspects of Design Space definition were investigated for manufacturing an OTC medicine. [1]
First, the relationship between granulator variables and the resulting granule quality, as quantified by the rheological properties, was investigated. Granules were generated using a pilot-scale HSWG process, then dried using a fluidised bed dryer before being milled, lubricated and finally tabletted. Immediately following the HSWG step, the dynamic, bulk and shear properties of the wet granules were measured using an FT4 Powder Rheometer® (Freeman Technology, UK).
The results of the first phase of the study (Figure 1) show that BFE of the wet granules can be predicted from knowledge of the process parameters, in particular Solid-to-Water Ratio and Impeller Speed. By adjusting these parameters operators can therefore attain a desired BFE value.
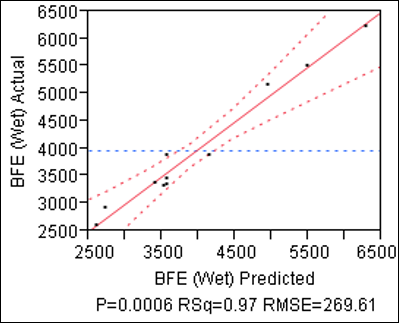
Figure 1: Actual v Predicted Basic Flowability Energy (BFE) values of Wet Granules
A second investigation was then carried out to determine how granule properties influenced CQAs of the resulting tablets.
Tablet hardness will depend on die fill depth, blend permeability and press pressure which are in turn influenced by variations in granule density, flow properties, compressibility and operating speed, among other things. To isolate the relationship between granule properties and tablet quality, an adjusted measure of the tablet hardness was adopted for this study to compensate for differences in process parameters.

Analysis of the tableting data (Figure 2) shows a strong relationship between BFE of the wet granules and Adjusted Hardness. The two studies combined therefore illustrate how CQAs of tablets can be achieved by controlling Critical Process Parameters.
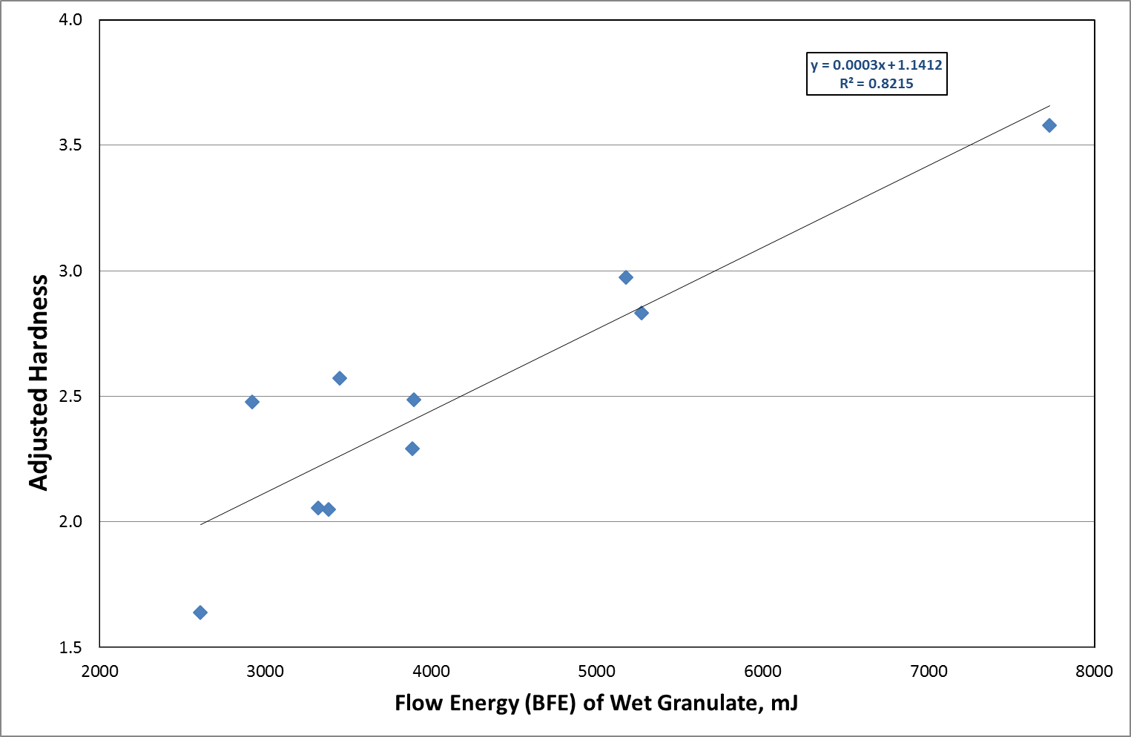
Figure 2: Tablet Hardness with respect to BFE of Wet Granules
Previous studies have also demonstrated the relationship between process variables and CQAs as well as how this approach can be applied to a continuous wet granulation process [2].
Developing a Design Space to Achieve CQA
Each stage of a process, from granulation to compression must function effectively to produce a quality product. Uncontrolled variation at any point in the manufacturing process can result in product failures and operational downtime. Robust Design Space definition provides operators with the opportunity to adjust settings to maintain quality. In this example, the BFE of wet granules has been shown to link directly to tablet quality. With sufficient understanding of the relevant material properties and Critical Process Parameters, it is possible to employ a QbD/Design Space approach to batch granulation and tablet manufacture, and convert potentially problematic upstream variation into an opportunity to optimise process performance and product quality.
References
1: T. Freeman, P. Kishinevskaya, J. Huang , M. Moshgbar, John Yin, Evaluating the Design Space for the Batch Manufacture of an OTC Medicine, , Freeman Technology, Pfizer Inc.
2: T. Freeman, A. Birkmire & B. Armstrong, A QbD Approach to Continuous Tablet Manufacture, Procedia Engineering, 102 (2015), pp443-449
Author Biography

Tim Freeman, Managing Director, Freeman Technology
Tim Freeman is Managing Director of powder characterisation company Freeman Technology for whom he has worked since the late 1990s. He was instrumental in the design and continuing development of the FT4 Powder Rheometer® and the Uniaxial Powder Tester. Through his work with various professional bodies, and involvement in industry initiatives, Tim is an established contributor to wider developments in powder processing.
Tim has a degree in Mechatronics from the University of Sussex in the UK. He is a mentor on a number of project groups for the Engineering Research Center for Structured Organic Particulate Systems in the US and a frequent contributor to industry conferences in the area of powder characterisation and processing. A past Chair of the American Association of Pharmaceutical Scientists (AAPS) Process Analytical Technology Focus Group Tim is a member of the Editorial Advisory Board of Pharmaceutical Technology and features on the Industry Expert Panel in European Pharmaceutical Review magazine. Tim is also a committee member of the Particle Technology Special Interest Group at the Institute of Chemical Engineers, Vice-Chair of the D18.24 sub-committee on the Characterisation and Handling of Powders and Bulk Solids at ASTM and a member of the United States Pharmacopeial (USP) General Chapters Physical Analysis Expert Committee (GC-PA EC).
-----------------------------------------------------------------------
Editor's Note:
If you have any suggestion to the content,
please email: Julia.Zhang@ubmsinoexpo.com.


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
Follow Us:




 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025


