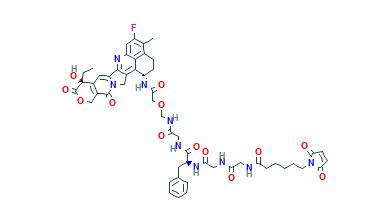
AstraZeneca and Daiichi Sankyo announced Wednesday that a pivotal Phase II study of trastuzumab deruxtecan in patients with refractory HER2-positive metastatic breast cancer met its primary endpoint, with the experimental drug demonstrating a clinically-meaningful response. The companies noted that results from the DESTINY-Breast01 trial are expected to support planned global regulatory submissions for trastuzumab deruxtecan in the second half.
In March, AstraZeneca agreed to pay Daiichi Sankyo $1.35 billion upfront as part of a deal potentially worth up to $6.9 billion to jointly develop and commercialise the HER2-targeting antibody-drug conjugate (ADC) trastuzumab deruxtecan. "We believe this antibody drug conjugate has the potential to redefine the treatment of patients with HER2-expressing cancers, and we are eager to bring it as quickly as possible to patients with refractory HER2-positive breast cancer who continue to have high unmet medical need," remarked José Baselga, executive vice president of R&D oncology at AstraZeneca.
The DESTINY-Breast01 study is a two-part trial evaluating trastuzumab deruxtecan in 253 patients with HER2-positive unresectable and/or metastatic breast cancer previously treated with Roche's ADC Kadcyla (trastuzumab emtansine). The study's primary endpoint is objective response rate, while secondary goals include duration of response, disease control rate, clinical benefit rate, progression-free survival and overall survival.
AstraZeneca and Daiichi Sankyo noted that the response rate seen in the trial, as assessed by an independent review committee, confirms in a heavily-pretreated patient population the clinical activity observed in the recently-published Phase I trial. The companies added that the safety of was consistent with the earlier study, in which the most common adverse events included nausea, decreased appetite, vomiting, alopecia, fatigue, anaemia, diarrhoea and constipation. Results from the Phase II trial are expected to be presented at an upcoming medical meeting.
Commenting on the news, Liberum analyst Alistair Campbell said "while positive data will have been expected given the Phase I data, this is good news for (AstraZeneca) as it adds validation to a major transaction that initially disappointed the market." Campbell estimates that trastuzumab deruxtecan could generate global sales of $5 billion annually.
Trastuzumab deruxtecan, also known as DS-8201, has been granted FDA breakthrough therapy designation and fast track status for HER2-positive patients in the advanced or refractory breast cancer setting. Pivotal studies of the therapy in HER2-expressing breast and gastric cancers, including in breast cancer patients with HER2-low expression, are under way, with mid-stage trials in patients with HER2-expressing advanced colorectal cancer and metastatic non-squamous HER2-overexpressing or HER2-mutated non-small-lung cancer also being conducted.
For related analysis, see ViewPoints: AstraZeneca speculates big to take on Roche and carve out a new breast cancer market.
Register as Visitor to CPhI China 2019!

-----------------------------------------------------------------------
Editor's Note:
To apply for becoming a contributor of En-CPhI.cn,
welcome to send your CV and sample works to us,
Email: Julia.Zhang@ubmsinoexpo.com





 ALL
ALL Pharma in China
Pharma in China Pharma Experts
Pharma Experts Market News
Market News Products Guide
Products Guide Brand Story
Brand Story

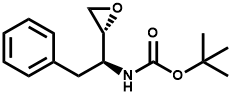
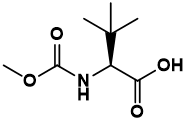



















 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025








