PharmaSources/CaicaiApril 03, 2019
Tag: china , sales , Osimertinib
The third generation has become the R&D hotspot of the giants. How Hot is the Third-generation EGFR-TKI?
Osimertinib, the only third-generation EGFR-TKI marketed in the world
Developed by AstraZeneca, osimertinib (trade name: Tagrisso, code: AZD9291) is the only third-generation EGFR-TKI marketed in the world at present, which mainly bind to the small T790M mutation domain through a smaller binding region, to reach the treatment effect. Its approval process is as shown below:
Nov. 2015 | Osimertinib received FDA’s accelerated approval to become the first targeted drug marketed for patients with T790M mutation-positive NSCLC who have progressed on or after EGFR-TKI therapy |
Mar. 2017 | Osimertinib received CFDA’s approval be formally marketed in China for adult patients with T790M mutation-positive locally advanced or metastatic NSCLC, as detected by test, who have progressed on or after EGFR-TKI therapy, being the first tumor drug approved in China for EGFR T790M mutation-positive locally advanced or metastatic NSCLC |
Sep. 2017 | NCCN guideline recommends osimertinib to be used as a first-line therapy for patients with locally advanced or metastatic EGFR mutation-positive NSCLC |
Apr. 2018 | FDA approved osimertinib as first-line therapy for patients with EGFR-mutation metastatic NSCLC |
June 2018 | EMA approved osimertinib as first-line therapy for patients with EGFR-mutation metastatic NSCLC |
Osimertinib has been approved in more than 80 countries including the U.S., EU, Japan, and China as a second-line therapy for patients with T790M resistance mutation NSCLC; and it has been approved in more than 60 countries as a first-line therapy since Apr. 2018, and it is expected to be approved in China in the first half of 2019.
Global sales doubled in 2018, sales in China of nearly RMB 2 billion
Osimertinib has emerged in the competition with each generation of TKI and its sales have rapidly grown, owing to the differentiation competitive advantage in T790M resistance mutation and outstanding clinical efficacy. Global sales of osimertinib reached USD 1.86 billion in 2018, growing by 95%; it has become the best-selling tumor drug of AstraZeneca and the drug with the second highest sales thereof, and it may become the best-selling drug of AstraZeneca in 2019, which is mainly because of its ascending into a first-line therapy and deeper penetration into more markets as a second-line therapy. Osimertinib’s sales reached RMB 1.85 billion in the first three quarters of 2018 in China, and its sales of the year approached RMB 2 billion.
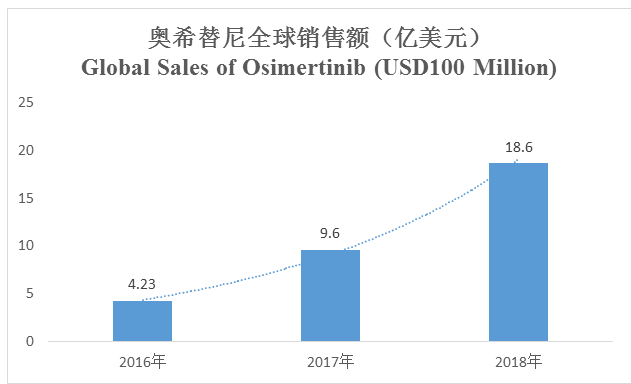
2016年、2017年、2018年 | 2016, 2017, 2018 |
(Source: Annual reports of the company)
Osimertinib has been included into Category B varieties of medical insurance in China in Oct. 2018, with the medical insurance payment standard of RMB 510 (80mg/tablet) and RMB 300 (40mg/tablet), and restricted coverage of adult patients with EGFR T790M mutation-positive locally advanced or metastatic NSCLC, as detected by test, who have progressed on or after EGFR-TKI therapy.
Read More:
How Hot is the Third-generation EGFR-TKI?
Caicai, a Master of Pharmacy from Shanghai Jiaotong University, used to work in the Institute of Science and Technical Information. Currently as a practitioner in the drug surveillance system, she is good at interpreting industry regulations, pharmaceutical research developments, etc.
-----------------------------------------------------------------------
Editor's Note:
To apply for becoming a contributor of En-CPhI.cn,
welcome to send your CV and sample works to us,
Email: Julia.Zhang@ubmsinoexpo.com.


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
Follow Us:




 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025


