PharmaSources/zhulikou431March 15, 2019
Tag: WHO , Pharmaceutical , Drug Supply Chain
There are about 700,000 people in the world who die of counterfeit drugs every year, according to statistics of the World Health Organization (WHO). And the annual losses of the pharmaceutical industry due to counterfeit drugs and patient safety issues reach up to USD200 billion. To curb such trends and ease concerns of patients, there have been many preventive measures introduced in the pharmaceutical industry:

EU:
As one of the strictest markets in terms of pharmaceutical product access requirements, the EU has been raising the thresholds in recent years, to prevent counterfeit drugs from flowing into the regular sales channels. In 2011, the EU made big amendment to the 2001 community code relating to medicinal products for human use (Directive 2001/83/EU), and issued the Directive 2011/62/EU, i.e., the EU Falsified Medicines Directive (EU FMD), to protect the pharmaceutical product supply chain from penetration by falsified (or counterfeit) drugs, and also introduced new rules to regulate the supply chain more strictly; the Official Journal of the European Union officially released the Directive 2016/161/EU on Feb. 9, 2016, which lays down in detail the safety features appearing on the packaging of medicinal products for human use, and has come into force on Feb. 9, 2019; and the newly upgraded version of the European Medicines Verification System (EMVS) that runs via the FMD has begun to be promoted in the Europe as of Feb. 9, 2019, which will be used by parties on the pharmaceutical product supply chain, including manufacturers, hospital supplies manufacturers, wholesalers, pharmacies, and hospitals. The system is connected to a trans-European database, to verify pharmaceutical products distributed in any place of the EU.
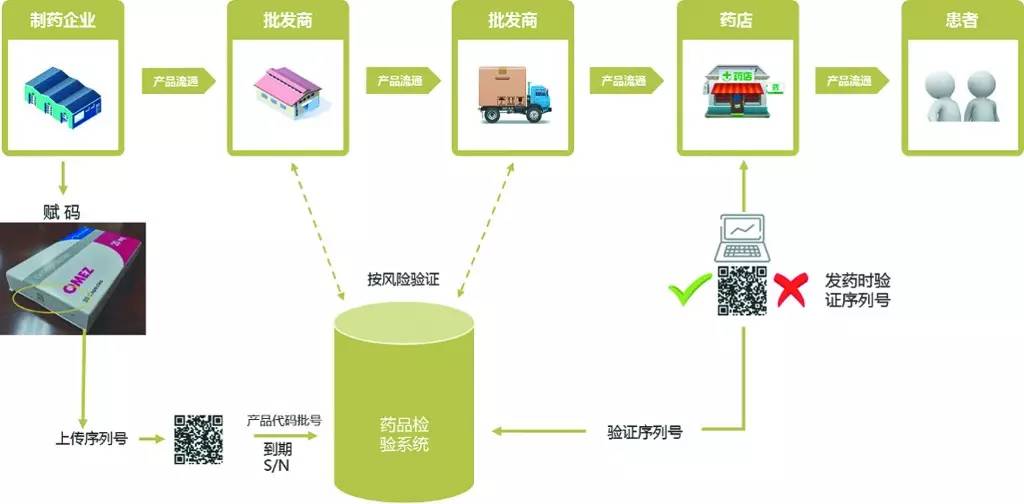
Fig. 1 Schematic Diagram of EMVS
制药企业 | Pharmaceutical manufacturer |
产品流通 | Product circulation |
批发商 | Wholesaler |
药店 | Pharmacy |
患者 | Patient |
赋码 | Unique serialization with random numbers |
上传序列号 | Upload number |
产品代码批号 | Product code batch |
到期S/N | Expiry S/N |
按风险验证 | Risk-based verification |
药品检验系统 | Medicines Verification System |
验证序列号 | Authenticate number |
发药时验证序列号 | Verification upon dispense to patient |
U.S.:
The Drug Supply Chain Security Act (DSCSA) has come into force in the U.S. on Nov. 27, 2013, which proposes requirements and steps for constructing an electronic supply chain tracking system and constructing of an electronic interoperable system that can identify and track some prescription drugs distributed in the U.S. DSCSA constructs a 10-year framework, with contents including drug traceability, product validation, and notification of stakeholders on prohibited drugs, etc. The relevant system must be implemented before Nov. 27, 2023. FDA released the Supply Chain Security Toolkit for Medical Products co-developed with the Asia-Pacific Economic Cooperation (APEC) and other parties in May 2017; FDA issued the draft guidance for industry: Product Identifier Requirements Under the Drug Supply Chain Security Act – Compliance on June 30, 2017, which described specific requirements for product identifiers.
The U.S. FDA announced on Feb. 7, 2019 that it would accept applications from the pharmaceutical industry and its supply chain partners for participating in the supply chain security pilot program, to test the use of product identifiers, bar codes, and interoperable systems for tracing and tracking prescription drugs in the whole industrial chain. FDA announced its plan for conducting the pilot program and solicited the industry opinions in 2017. According to FDA, "The pilot program will be designed to explore issues related to utilizing the product identifier for product tracing, improving the technical capabilities of the supply chain, identifying the (electronic) system attributes that are necessary to implement the requirements established under the DSCSA."
Guaranteeing the traceability of pharmaceutical products is one of the key factors for fighting counterfeit drugs. The fundamental goal of the U.S. DSCSA and EU FMD is to establish a safer pharmaceutical product supply chain, and both sides are pushing ahead with it to ensure the security thereof.
Reference:
Zhulikou431, as a senior engineer, PDA member, ISPE member, ECA member, PQRI member, senior aseptic GMP expert, has deep knowledge in aseptic process development and verification, drug development and registration, CTD document writing and review, regulatory audit, international certification, international registration , quality system construction and maintenance, as well as sterile inspection, environmental monitoring and other fields. In recent years, he has focused on the analysis of trends in the macro pharmaceutical field and the risk management of pharmaceutical enterprise mergers and acquisitions projects.
-----------------------------------------------------------------------
Editor's Note:
To apply for becoming a contributor of En-CPhI.cn,
welcome to send your CV and sample works to us,
Email: Julia.Zhang@ubmsinoexpo.com.


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
Follow Us:




 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025


