PharmaSources/1℃March 11, 2019
Tag: AbbVie , Mavyret , HCV Drug Market , DAAs
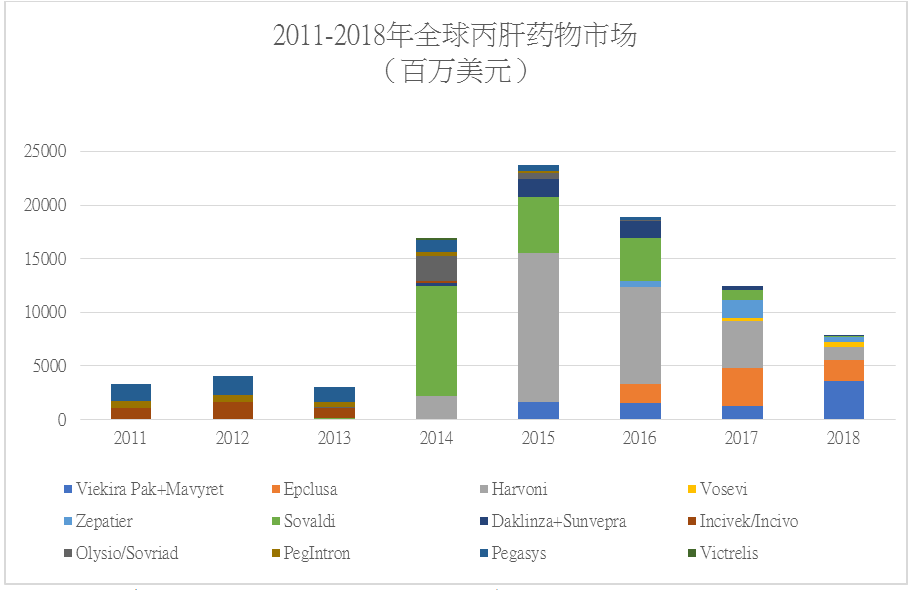
The global HCV drug market continued to shrink in 2018. According to the financial reports of the companies, the global HCV drug market reached USD7.767 billion in 2018, down by 37.75% year on year, and by USD15.996 billion (-67.32%) compared to the peak of the global HCV drug market in 2016.
Combination regimens of direct-acting antivirals (DAAs) and all-oral DAA regimens have increased the SVR to 90%-100%, which is revolutionary progress. DAAs have opened the era of hepatitis C cure. The development trends of the global HCV drug market from 2011 to 2018 are reviewed next in this article.
I. Hepatitis C cure: DAAs have opened a new era
J & J launched simeprevir in 2013;
Gilead launched sofosbuvir, the first generation, in 2013!
And the era of DAAs has begun!
Contributions of DAAs are epochal!
DAAs + Peg-IFN-α+ ribavirin or all-oral DAA regimens have increased the SVR of HCV patients to 90%-100%, i.e., cure of HCV!
Since then, Gilead, J & J, AbbVie, BMS, and MSD have successively launched many DAAs, with each product leading for 1-2 years!
Remarks: The market data of the interferon product including sales of indications including HCV and HBV, etc.
Gilead’s Sovaldi, Harvoni, and Epclusa, and AbbVie’s Mavyret were the most successful among the DAAs, wherein, Harvoni’s sales peaked at USD13.864 billion!
Mavyret outshone amid the big decline of the HCV drug market, which was marketed in 2017, with a shorter treatment cycle (the only DAA with treatment course of 8 weeks) and largely reduced treatment expenses. Its sales performance has skyrocketed since marketed!
Quarterly performance of main products in the HCV drug market in 2017-2018:
Performance of Viekira Pak +Mavyret has reached the ceiling
Performance of Viekira Pak +Mavyret continued to rise sharply in 2018 compared to 2017, however, the quarterly performance thereof has reached the ceiling and started to decline, wherein, the growth drivers have almost disappeared in the U.S. market, and the future sales growth depends on the international market (especially China). According to my forecast, the 2019 sales thereof will be about USD3.1 billion!
Performance of Gilead’s Epclusa continued to decline:
Gilead’s Harvoni: twilight of the king
II. Post-DAA era: AbbVie’s Mavyret to become the terminator
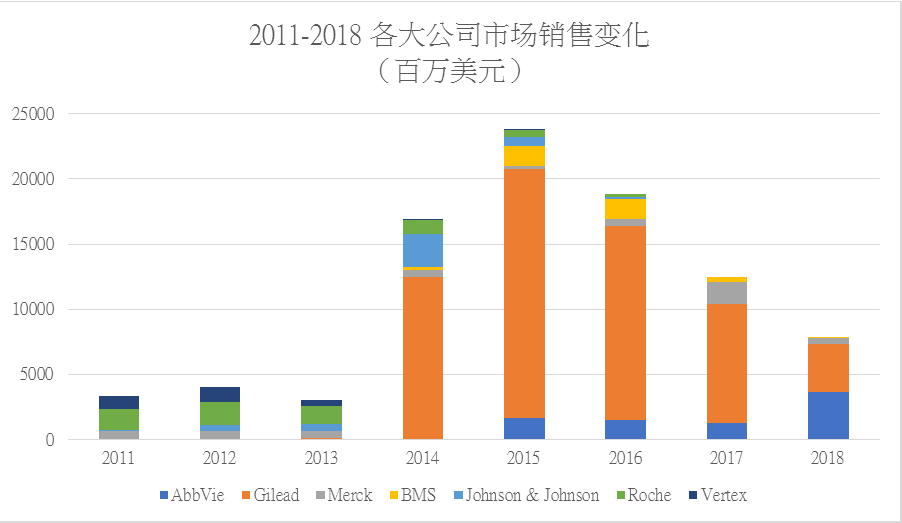
2014-2017: Gilead was the unshakeable king in the global HCV drug market
2014-2015: Gilead advanced triumphantly
Gilead was invincible in the HCV drug market with Sovaldi, Harvoni, and Epclusa, etc., and its HCV drug business reached the record-breaking USD19.1 billion in 2015;
2016-2017: Gilead carried out self-revolution
The market rapidly shrank owing to the HCV particularity and unprecedentedly high cure rate of DAAs. Gilead’s HCV drug business continued to largely decline in 2016 and 2017!
2018: AbbVie’s HCV drug bucked the trend and rose, and Mavyret became the terminator
In 2018, the dominant position of Gilead in the HCV drug market disappeared, and AbbVie caught up from behind. According to data, 2018 income of the HCV drug business of Gilead reached USD3.686 billion, while that of AbbVie reached USD3.616 billion; Gilead won AbbVie by a narrow margin. However, 2019 income of the HCV drug business of AbbVie is expected to be USD3.1 billion, and that of Gilead is expected to be USD2.8 billion!
III. China to enter the era of hepatitis C cure
China will enter the era of hepatitis C cure by following closely the EU and U.S. There have been 8 DAA regimens approved n China, and 3 DAAs in the marketing review stage; all of them are expected to be approved for marketing in 2019, including AbbVie’s Maviret.
According to the prices released by the enterprises so far:
1. Prices of imported drugs are between RMB58,000-60,000/12 weeks
2. Prices of Chinese-produced drugs are about RMB40,000/12 weeks
China will enter the new era of complete cure with DAAs + Peg-IFN-α+ ribavirin or all-oral DAAs, with the approval for marketing of DAAs in China.
Trade name | Generic name | Therapeutic regimen | China | Company |
Daklinza | Daclatasvir | DCV+ASV, 24 weeks | Apr. 24, 2017 | BMS |
Sunvepra | Asunaprevir | |||
Olysio | Simeprevir | SMV, 24/48 weeks | Aug. 24, 2017 | J & J |
Viekirax | Ombitasvir / paritaprevir / ritonavir | OBV/PTV+DSV, 12 weeks | Sep. 21, 2017 | AbbVie |
Exviera | Dasabuvir | Sep. 21, 2017 | ||
Sovaldi | Sofosbuvir | SOF+PR, 12 weeks | Sep. 21, 2017 | Gilead Sciences |
SOF+RBV, 12/24 weeks | ||||
Zepatier | Elbasvir Grazoprevir | EBR/GRZ, 12 weeks | Apr. 28, 2018 | Merck |
Epclusa | Sofosbuvir Velpatasvir | SOF+VEL, 12 weeks | May 30, 2018 | Gilead Sciences |
Ganovo | Danoprevir | Danoprevir + PR, 12 weeks | June 13, 2018 | Ascletis |
Harvoni | Ledipasvir Sofosbuvir | LDV+SOF, 12 weeks | Dec. 04, 2018 | Gilead Sciences |
Maviret | Glecaprevir Pibrentasvir | GLE+PIB, 8 weeks | Applied for marketing | AbbVie |
| KW-136 | KW-136+SOF | Applied for marketing | Kawin Technology |
| Ravidasvir | Ravidasvir + danoprevir | Applied for marketing | Ascletis |
-----------------------------------------------------------------------
Editor's Note:
To apply for becoming a contributor of En-CPhI.cn,
welcome to send your CV and sample works to us,
Email: Julia.Zhang@ubmsinoexpo.com.


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
Follow Us:




 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025


