PharmaSources/CaicaiJanuary 21, 2019
Tag: 2019 , Chinese-produced Drug
Chinese-produced new drugs to be marketed soon in 2019:
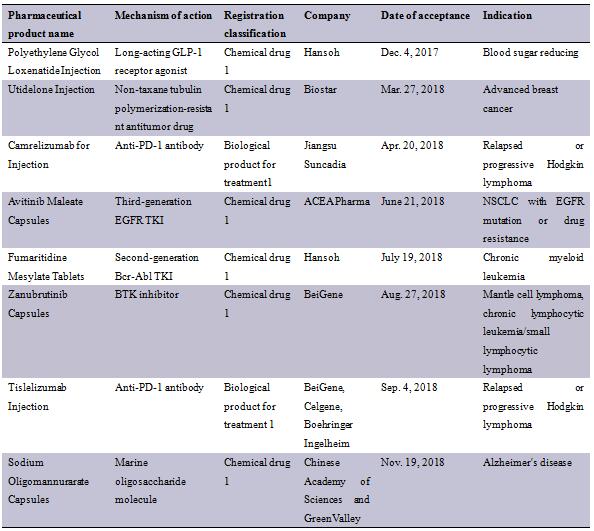
Pharmaceutical product name | Mechanism of action | Registration classification | Company |
Polyethylene Glycol Loxenatide Injection | Long-acting GLP-1 receptor agonist | Chemical drug 1 | Hansoh |
Utidelone Injection | Non-taxane tubulin polymerization-resistant antitumor drug | Chemical drug 1 | Biostar |
Camrelizumab for Injection | Anti-PD-1 antibody | Biological product for treatment1 | Jiangsu Suncadia |
Avitinib Maleate Capsules | Third-generation EGFR TKI | Chemical drug 1 | ACEA Pharma |
Fumaritidine Mesylate Tablets | Second-generation Bcr-Abl TKI | Chemical drug 1 | Hansoh |
Zanubrutinib Capsules | BTK inhibitor | Chemical drug 1 | BeiGene |
Tislelizumab Injection | Anti-PD-1 antibody | Biological product for treatment 1 | BeiGene, Celgene, Boehringer Ingelheim |
Sodium Oligomannurarate Capsules | Marine oligosaccharide molecule | Chemical drug 1 | Chinese Academy of Sciences and GreenValley |
(Sorted out based on public data. Any supplementation will be much appreciated. *Jiangsu Suncadia is a subsidiary of Hengrui Medicine)
Caicai, a Master of Pharmacy from Shanghai Jiaotong University, used to work in the Institute of Science and Technical Information. Currently as a practitioner in the drug surveillance system, she is good at interpreting industry regulations, pharmaceutical research developments, etc.
-----------------------------------------------------------------------
Editor's Note:
To apply for becoming a contributor of En-CPhI.cn,
welcome to send your CV and sample works to us,
Email: Julia.Zhang@ubmsinoexpo.com.


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
Follow Us:




 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025


