Tim FreemanJanuary 14, 2019
Tag: capsule filling , Tim Freeman , Powder Flow and Powder Testing
In the pharmaceutical industry, the blending of a small fraction of active pharmaceutical ingredient (API) with a bulk excipient, and then subsequent processing though various unit operations, is an essential part of many manufacturing processes. The way that the blend behaves in downstream processes will be a function of the properties of the components of that blend.
When manufacturing formulations that will be delivered in capsule form, the blend requires suitable characteristics to ensure a precise and accurate fill of the dosing capsule. The ability to characterise formulations with respect to process behaviour is an essential part of Quality by Design (QbD). A method of quantifying the properties of powdered formulations that are conducive to good performance in a dosator, allows a design space to be defined, specifying material properties that will result in high quality final products. This provides significant commercial benefits in terms of higher productivity and reduced wastage.

Performance Variation Between Similar Formulations
Three powdered formulations were used in the production of a DPI product. Differences in the quality of the final product were observed depending on which formulation was used. Of the three formulations, Formulation 1 represented average behaviour in terms of filling performance from the dosator, whereas Formulation 2 represented the worst behaviour, and Formulation 3 the best.
Samples from the three formulations were evaluated using an FT4 Powder Rheometer®. The tests investigated the Dynamic, Bulk and Shear properties of the samples, in order to identify trends that correlated with the dosator performance.
Test Results
Dynamic Testing: Specific Energy
Formulation 2 had the highest Specific Energy of the three materials, and Formulation 3 the lowest. High Specific Energy represents a greater degree of mechanical interlocking and friction within the bulk, typically leading to problems in operations such as filling, where gravitational flow is critical.
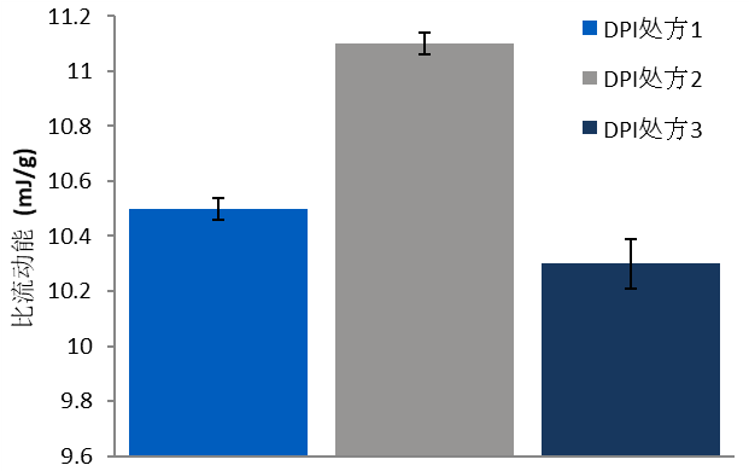
Figure 1
Bulk Testing: Compressibility
Formulation 2 was the most compressible of the samples, and Formulation 3 the least. High Compressibility indicates that a powder entrains a greater proportion of air within its bulk, which is a property typically associated with more cohesive powders.
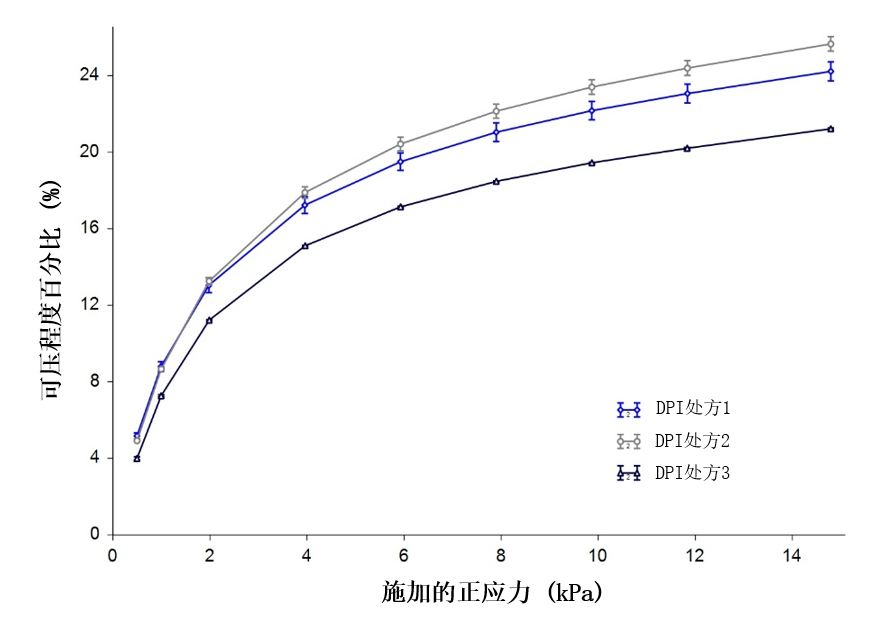
Figure 2
Shear Cell Testing
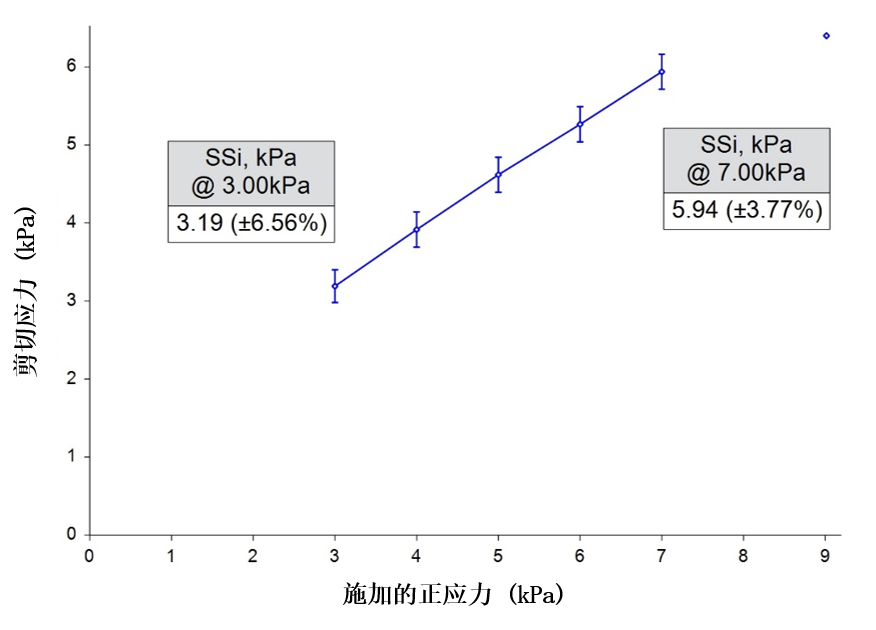
Figure 3
Minimal differentiation was observed between the three samples, with six data sets, i.e. two repeats on each sample, generating near identical Shear Stress values to within an acceptable standard deviation. This lack of correlation with the process behaviour illustrates how Shear Cell testing, designed to investigate how a powder transitions from a static to dynamic state following consolidation, may not be relevant to the more dynamic, low-stress conditions that a powder is exposed to in the dosing process.
Conclusions
The FT4 has identified clear and repeatable differences between three similar formulations which correlated well with the relative performance observed in the dosator. Specific Energy and Compressibility were shown to be highly differentiating suggesting that mechanical interlocking/friction and packing structure of the powder bed have the greatest influence on process performance. Limited information was provided by the Shear Cell test, suggesting that shear properties had little or no influence on the overall performance. The Dynamic and Bulk parameters can be used to construct a design space of process-relevant measurements, which can be used as a template for future formulations and enable process behaviour to be predicted with confidence.
Powder flowability is not an inherent material property, but is more about the ability of powder to flow in a desired manner in a specific piece of equipment. Successful processing demands that the powder and the process are well-matched and it is not uncommon for the same powder to perform well in one process but poorly in another. This means that several characterisation methodologies are required, the results from which can be correlated with process ranking to produce a design space of parameters that correspond to acceptable process behaviour. Rather than relying on single number characterisation to describe behaviour across all processes, the FT4’s multivariate approach simulates a range of unit operations, allowing for the direct investigation of a powder’s response to various process and environmental conditions.
Author Biography -

Tim Freeman, Managing Director, Freeman Technology
Tim Freeman is Managing Director of powder characterisation company Freeman Technology for whom he has worked since the late 1990s. He was instrumental in the design and continuing development of the FT4 Powder Rheometer® and the Uniaxial Powder Tester. Through his work with various professional bodies, and involvement in industry initiatives, Tim is an established contributor to wider developments in powder processing.
Tim has a degree in Mechatronics from the University of Sussex in the UK. He is a mentor on a number of project groups for the Engineering Research Center for Structured Organic Particulate Systems in the US and a frequent contributor to industry conferences in the area of powder characterisation and processing. A past Chair of the American Association of Pharmaceutical Scientists (AAPS) Process Analytical Technology Focus Group Tim is a member of the Editorial Advisory Board of Pharmaceutical Technology and features on the Industry Expert Panel in European Pharmaceutical Review magazine. Tim is also a committee member of the Particle Technology Special Interest Group at the Institute of Chemical Engineers, Vice-Chair of the D18.24 sub-committee on the Characterisation and Handling of Powders and Bulk Solids at ASTM and a member of the United States Pharmacopeial (USP) General Chapters Physical Analysis Expert Committee (GC-PA EC).
www.freemantech.com.cn
info@freemantech.com.cn
Register as Visitor to CPhI China 2019!
-----------------------------------------------------------------------
Editor's Note:
To apply for becoming a contributor of En-CPhI.cn,
welcome to send your CV and sample works to us,
Email: Julia.Zhang@ubmsinoexpo.com.


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
Follow Us:




 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025


