pharmaceutical-technologyJanuary 08, 2019
Tag: immunotherapy , Cancer vaccines , HPV
Cancer vaccines are a type of immunotherapy used to either treat existing cancer or prevent the development of cancer.
Cancer treatment vaccines, also called therapeutic vaccines, work to boost the body’s natural defences in fighting cancer and may stop the growth of cancer, prevent recurrence or destroy cancer cells remaining after other treatments.
Therapeutic cancer vaccines boost the immune system’s ability to recognise and destroy antigens. Cancer cells often have certain molecules called tumour-associated antigens on their surface that healthy cells do not have.
When these vaccines are given to a person, the molecules act as antigens. They stimulate the immune system to recognise and destroy cancer cells that have these molecules on their surface.
Most cancer vaccines also contain adjuvants, which are substances that may help strengthen the immune response.
Some cancer vaccines are produced from the patient’s tumour sample, others target tumour-associated antigens and are given to patients whose tumours have been found to have those antigens on the surface of the tumour cells.
The most commercially successful type of cancer vaccines are those that prevent cancer from developing, also known as prophylactic vaccines.
There are two types approved by the FDA: human papillomavirus (HPV) vaccines approved for the prevention of anal, cervical, vaginal and vulvar cancer, and hepatitis B vaccines that can prevent liver cancer.
Data Source: GBI Research
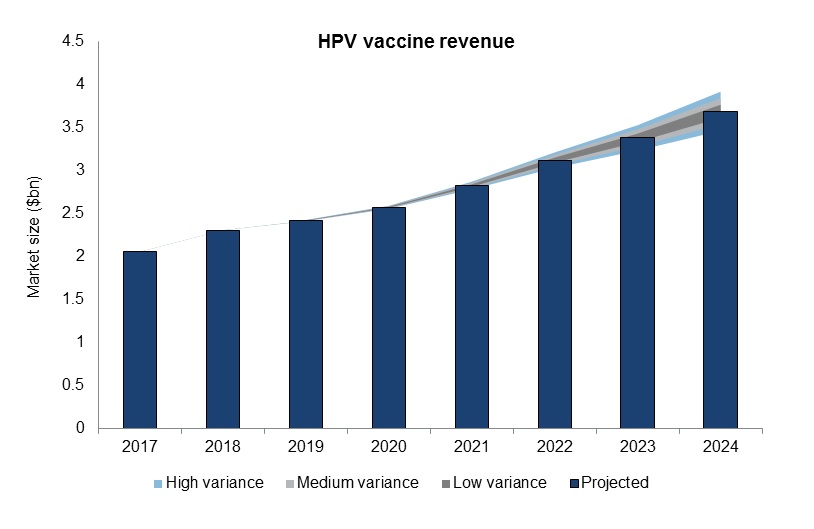
Only one cancer vaccine achieved blockbuster revenue status in 2017, Gardasil, a prophylactic vaccine marketed by Merck that inoculates against HPV.
Gardasil and other HPV vaccine-associated revenue are expected to experience continued growth, from $2.1bn in 2017 to $3.7bn in 2024, at a CAGR of 8.7%, accounting for 95% and 82% of total cancer vaccine revenue in 2017 and 2024, respectively.
While the cancer vaccines therapy area is dominated commercially by HPV vaccines, there remains a strong interest in developing therapeutic cancer vaccines, with a considerable 966 cancer vaccines in the pipeline.
Research in this area remains at an early stage, and it has proven difficult for the vast majority of cancer vaccines to gain regulatory approval, and the therapy area is associated with a high attrition rate.
However, in 2010, the FDA approved Provenge (sipuleucel-T) for men with metastatic prostate cancer, and more cancer vaccines are anticipated to gain approval before 2024.
Register as Visitor to CPhI China 2019!
-----------------------------------------------------------------------
Editor's Note:
To apply for becoming a contributor of En-CPhI.cn,
welcome to send your CV and sample works to us,
Email: Julia.Zhang@ubmsinoexpo.com.


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
Follow Us:




 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025


