PharmaSources/1℃January 03, 2019
Tag: FDA , drug review , approvals , noval drugs , 2018
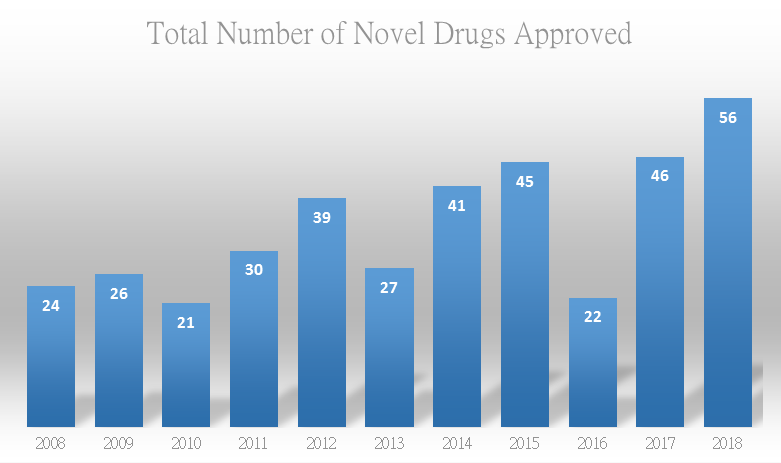
Looking back 2018, FDA has started to implement the real-time review, and implemented strict review of opioids. Among the drugs approved, cannabidiol, lofexidine, 3 anti-CGRP monoclonal antibody (mAb) drugs, the first RNAi drug, and small molecule broad-spectrum anticancer drug, etc. are especially impressive; rare disease Lambert-Eaton myasthenic syndrome, hereditary angioedema, Dravet syndrome, and Fabry disease, etc. usher in innovative therapies; FDA approved the world’s 6th anti-PD-(L)1 mAb, and HIV cocktail therapy and antibody drugs obtain breakthrough progress, etc.
Looking back 2018, the marketing of many drugs is of great significance. Here, I’d like to review several of them for your discussion and reference.
I. Migraine antibody drugs: 3 anti-CGRP mAbs approved for marketing
Anti-CGRP mAbs are a variety of drugs with quite definite mechanism of action. Amgen/Novartis, Teva, and Eli Lilly have successively marketed 3 anti-CGRP mAbs in 2018, showing breakthrough progress of migraine drugs. The 3 drugs enjoy positive market expectations, and are injected subcutaneously and priced at $6,900/year. However, the medication regimen of Teva’s drug is more flexible: it can be used by month/quarter. According to the forecast of Evaluate Pharma, the anti-CGRP drug market can reach USD5 billion in 2024. Refer to the article Global CGRP Drug Market Expected to Reach USD5 Billion by 2024, with 4 Anti-CGRP Antibodies Ranking Top 4 of the Market for the details.
Wherein, Aimovig (erenumab) of Novartis/Amgen was the first approved for marketing and became the first in class, which is one of the most noteworthy review developments in 2018; sooner or later, the drug will grow into a blockbuster drug as it could bring significant clinical benefits to migraine patients. According to the data of NCT02066415 clinical trial, compared to the placebo, the drug could reduce the monthly attacks of chronic migraine patients further by 2.5 attacks.
This area still merits attention in 2019, with the focuses mainly on: 1. The antibody drug eptinezumab developed by Alder will be approved for marketing to become the 4th anti-CGRP antibody; 2. The small molecule anti-CGRP drug: Ubrogepant developed by Allergan will also be filed the marketing application, to hopefully become the first small molecule anti-CGRP drug approved for marketing.
II. The small molecule broad-spectrum anticancer drug: Vitrakvi to continue to expand the pan-cancer therapy
In 2017, keytruda received an indication of concern, i.e., MSI-H/dMMR solid tumors. Its regimen to prevent and treat multiple cancers "in a basket" became a much concerned focal topic, and the "Basket Trial" gained extensive attention.
Loxo Oncology continued its legend on Nov. 26, 2018: Vitrakvi (larotrectinib) was approved for advanced solid tumors harboring an NTRK gene fusion, making larotrectinib (LOXO-101), a legendary molecule that shone in 2017 ASCO, attract the world’s attention again. The clinical data showed that the drug reached ORR of 78% among 55 patients with salivary gland cancer, carcinosarcoma, infantile fibrosarcoma, lung cancer, or thyroid cancer, etc.
Vitrakvi, despite a very small market, will make "precision medicine" and "broad-spectrum anticancer" more deeply into people’s minds and the intensive and more precise patient stratification become clearer in the future as a small molecule broad-spectrum anticancer drug.
III. The world’s 6th anti-PD-(L) 1 antibody: Libtayo® accelerated the marketing
There have been 6 anti-PD-(L)1 antibodies marketed in the world by Dec. 16, 2018, including MSD’s Keytruda, and BMS’ Opdivo, etc.
Sanofi/Regeneron announced on Sep. 28, 2018 that FDA approved the marketing of Libtayo® (cemiplimab-rwlc), which becomes the one and only drug approved for metastatic/locally advanced cutaneous squamous cell carcinoma (CSCC). Cemiplimab has been completed marketing with CSCC as the entry point, and will subsequently focus on the indication of non-small cell lung cancer (NSCLC).
In 2019, MSD’s Keytruda and BMS’ Opdivo will continue to maintain their industry leading positions worldwide; the market sales of Roche’s Tecentriq will be significantly accelerated owing to the breakthroughs as a first-line therapy for triple negative breast cancer and for NSCLC, and will leave relevant drugs of AstraZeneca and Merck/Pfizer far behind.
In 2019, the number of Chinese manufacturers and medical product suppliers developing anti-PD-(L)1 antibody will reach over 30, and there will be many Chinese-produced/imported anti-PD-(L)1 antibodies approved for marketing in China; indications of Keytruda/Opdivo will further increase; development of anti-PD-(L)1 antibodies will become increasingly refined, and patient stratification will increasingly tend to be niche and precise. How to fight for a piece of world in the niche area, and how to cleverly develop the drug combination regimens will be an important question that later anti-PD-(L)1 antibodies need to answer.
IV. Another breakthroughs of HIV cocktail therapy and antibody drugs
HIV prevention and treatment is a global issue. Cocktail therapies have started the new generation of HIV therapeutic regimens. The global HIV market size approached USD23.5 billion in 2017, and Gilead dominates the global HIV drug market with absolute advantages.
1. Gilead launched another single table regimen in 2018: Biktarvy based on basic components of TAF
Gilead occupies half of the global HIV drug market. We can see from its financial report data that the new generation of HIV drugs developed based on TAF represented by Genvoya, Odefsey, and Descovy have continued to enjoy sharp rise, and predictably, the market sales of Biktarvy are also positive and optimistic.
2. Targeting drug resistant HIV: Trogarzo (ibalizumab-uiyk) is a breakthrough development of HIV drugs
The ibalizumab of TaiMed Biologics, an anti-CD4 antibody, has received much attention since marketed. The drug is the first in class. The creation of the cocktail therapies and the marketing of integrase inhibitor drugs in recent years make HIV become chronic disease, however, multi-drug resistant HIV patients have no access to drugs. Ibalizumab focuses on the high tolerant HIV patients, and its development and marketing are an important breakthrough in recent decades.
Furthermore, MSD’s HIV drug Delstrigo (doravirine 100 mg/ TDF 300 mg/ 3TC 300 mg) was approved for marketing, which is another 2NRTI/NNRTI drug.
V. Marketing of Lucemyra under the background of strict review of opioids
In 2017 when Scott Gottlieb started to take office as FDA commissioner, he proposed to strictly control opioid abuse and start strict review against opioid abuse in his first address. And Opana ER (oxymorphone preparation) was ordered to be withdrawn from the market.
Opioids are powerful analgesics. Under the background of strict review against drug abuse, any non-opioid drug that can effectively control or mitigate opioid drug abuse may become a hotspot.
As a result, we can understand the significance of the marketing of Lucemyra (lofexidine). Lofexidine was not first marketed in the U.S., and it was rapidly marketed in the U.S. through priority review + fast track, to become the first non-opioid drug approved by FDA for opioid withdrawal syndrome.
VI. Epidiolex: Magical marijuana extract
Epidiolex (cannabidiol) developed by GW Pharma was approved by FDA on June 25, 2018, with the drug indications of Lennox-Gastaut syndrome and Dravet syndrome.
According to 3 key clinical data, 1. Epidiolex could significantly reduce the number of drop seizure and total seizure in patients with moderate Lennox-Gastaut syndrome, and GWPCARE3 clinical data showed that 2 doses of Epidiolex (20mg/kg/day, 10mg/kg/day) could both significantly improve clinical symptoms of seizure patients; 2. The dose of 20mg/kg/day of Epidiolex could significantly reduce convulsions of patients with Dravet syndrome, and could effectively reduce seizures during the treatment and maintenance periods.
GW Pharma is a unique company that has a very deep understanding of cannabinoid. Epidiolex was approved after the successful launch of Sativex, and has brought a new treatment choice for patients with the rare diseases of Lennox-Gastaut syndrome or Dravet syndrome.
VII. Milestone meaning: RNAi drug Onpattro approved for marketing
The RNAi drug Onpattro (patisiran) of Alnylam/Sanofi was approved for marketing on Aug. 10, 2018, with the indication of polyneuropathy of hereditary transthyretin-mediated amyloidosis.
RNAi drug patisiran is a drug that cannot be ignored in 2018. The marketing of patisiran as the first in class has successfully commercialized RNAi technique, showing how important the drug is. Here, I will not go into the technical details of the drug. More information can be learned on the website of Alnylam.
VIII. IDH1 inhibitor and epigenetics: Tibsovo (ivosidenib)
The successive marketing of two IDH inhibitors of Agios Pharmaceuticals has attracted more attention of the industry to epigenetics and IDH pathway, etc. Epigenetics has been a hot research field; isocitrate dehydrogenase includes IDH1, IDH2, and IDH3; IDH plays an important role in tricarboxylic acid cycle and can be catalyzed into α-Ketoglutaric acid, which is closely linked with key epigenetics such as histone modification and DNA methylation.
IDH is identified in many tumor types, wherein, the IDH mutant (IDHm) accounts for about 20% in AML patients. Refer to the following table for the statistical data of other tumor types:
European and America | IDH1/2m, % | IDH1/2m, number of people |
AML | IDH1/2m, 20% | IDH1/2m, 10,000 |
Cholangiocarcinoma | IDH1m, 14% | IDH1m, 3,000 |
Myelodysplastic syndrome | IDH1/2m, 8% | IDH1/2m, 3,000 |
IDH inhibitors provide a novel therapeutic regimen for cancer patients. There are also Chinese enterprises focusing on development of such drugs, for example, Cstone Pharmaceuticals has obtained the rights and benefits in ivosidenib in Greater China, and the Chinese enterprise Nanjing Sanhome’s SH1573 is in IND review stage.
The attached table shows the novel drugs marketed in 2018.
-----------------------------------------------------------------------
Editor's Note:
To apply for becoming a contributor of En-CPhI.cn,
welcome to send your CV and sample works to us,
Email: Julia.Zhang@ubmsinoexpo.com.


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
Follow Us:




 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025


