The Chinese API maker at the heart of a global scare and recall of blood pressure medicines has been savaged in an FDA warning letter for failing to uncover a suspected carcinogen in its APIs when a customer complained several years ago.
The FDA said that when Zhejiang Huahai Pharmaceutical altered its manufacturing process in 2011 to include a solvent suspected of producing the impurity, it didn’t even consider that the changes might lead to the formation of mutagenic impurities in its valsartan APIs.
"You failed to adequately assess the potential formation of mutagenic impurities when you implemented the new process," the highly redacted warning letter says.
FREE DAILY NEWSLETTER
Like this story? Subscribe to FiercePharma!
Biopharma is a fast-growing world where big ideas come along daily. Our subscribers rely on FiercePharma as their must-read source for the latest news, analysis and data on drugs and the companies that make them. Sign up today to get pharma news and updates delivered to your inbox and read on the go.
SUBSCRIBE NOW
That criticism of the Chuannan plant comes despite the fact that the FDA has admitted that it was unaware that certain manufacturing processes under certain conditions would result in the formation of N-nitrosodiethylamine and N-nitrosodimethylamine during manufacturing of "sartan" products. The two probable carcinogens were discovered in valsartan APIs produced by Huahai but have since been found in the APIs of other drugmakers, including Aurobindo and Mylan, and in finished products from Sandoz and Teva.
RELATED: FDA berates Chinese drugmaker tied to global valsartan recall
The initial discovery of one of the impurities this summer by a U.S. drug manufacturer that had used Huahai’s valsartan API set off the recall across the globe of hundreds of lots of valsartan, irbesartan and losartan, ubiquitous drugs used to lower blood pressure.
While the impurities have been found in the APIs of other drugmakers, the FDA warning letter suggests that the formation of the impurities could have been uncovered and resolved at least two years ago. It indicates Huahai did not take seriously a complaint from a customer whose own testing found one of the carcinogens in an API that Huahai had indicated passed testing. The FDA says Huahai didn’t even bother to test other batches to see if the existence of the potentially dangerous contaminant was an "adverse trend."
RELATED: Valsartan recall expands to Mylan, Teva products
Instead, the FDA says Huahai accepted the returned batches, then "reprocessed and released [them] to customers in non-U.S. markets."
The agency has banned APIs coming out of Huahai’s manufacturing site in Chuannan and earlier lambasted it in an 11-observation Form 483 for making manufacturing process changes without conducting proper risk assessments.
Regulators in the U.S. and Europe continue to test products to try to ferret out all of the affected drugs. The FDA says there is very little risk of the impurities causing problems, and no adverse reactions have been seen. Still, they continue to ask drugmakers to pull their products off the market when impurities are detected. There are still lots of blood pressure medications that have been unaffected by the recalls, according to a list (PDF) maintained by the agency.
-----------------------------------------------------------------------
Editor's Note:
To apply for becoming a contributor of En-CPhI.cn,
welcome to send your CV and sample works to us,
Email: Julia.Zhang@ubmsinoexpo.com.





 ALL
ALL Pharma in China
Pharma in China Pharma Experts
Pharma Experts Market News
Market News Products Guide
Products Guide Brand Story
Brand Story


















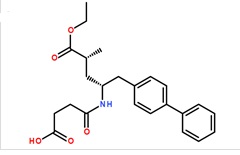




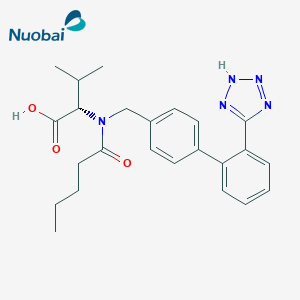



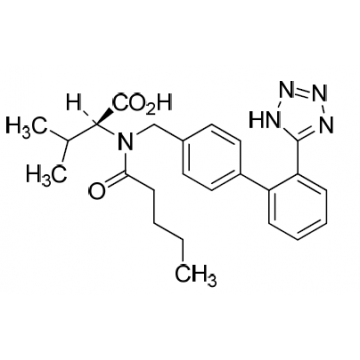





 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025








