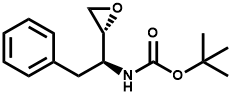
Hengrui announced at the end of last month that it had received the Drug Clinical Trial Approval issued by the State Drug Administration of China (SDA) and would conduct Phase I clinical trial soon. The basic information of the pharmaceutical product is as follows:
|
Pharmaceutical product name
|
SHR-1603 Injection
|
|
Dosage form
|
Injection
|
|
Specification
|
2ml:0.1g
|
|
Application matter
|
Registration of Chinese-produced pharmaceutical product
|
|
Registration classification
|
Biological product for treatment
|
|
Applicant
|
Jiangsu Hengrui Medicine Co., Ltd., Shanghai Hengrui Pharmaceutical Co., Ltd., and Suzhou Suncadia Biopharmaceuticals Co., Ltd.
|
|
Acceptance No.
|
CXSL1800011
|
|
Approval No.
|
2018L02559
|
(Source: Eastmoney.com)
The Chinese R&D giant Hengrui has kept this pharmaceutical product in strict confidence, which only has a code and does not provide any information about the target, therefore, this news did not cause much backlash in the industry at that time. However, I’ve learned from an insider recently that SHR-1603 actually targets the very hot oncology target CD47 in recent two years. You don’t know what CD47 is? Let me analyze it below.
Anti-CD47 antibody—the next star in the tumor immunotherapy field
CD47 is also known as integrin associated protein (IAP) that belongs to the immunoglobulin superfamily; the protein is a five transmembrane protein, with molecular weight of about 50kDa, and extracellular N-terminal of IgG4 domains. CD47 is an important marker of "self" on the cell surface and an important signal regulating the phagocytosis of macrophages; it can bind to SIRPα on the surface of macrophages and phosphorylate the ITIM thereof, and then recruit SHP-1 protein, to produce a series of cascade reactions to inhibit the phagocytosis of macrophages.
CD47 is extensively expressed on the surface of many healthy cells and releases "Don't eat me" signal to macrophages, thus to protect healthy cells from damaging by the immune system. When cells age or have cytopathy, cell surfaces will gradually lose CD47 to help the body identify and deal with those cells. Unfortunately, this mechanism is used by the cancer cells which, in order to protect themselves, arrange more CD47 on their surface than normal cells have, and as CD47 binds to SIRPα, the "Don't eat me" is also released, resulting in the macrophages not to swallow the cancer cells.
There are currently two theories regarding the mechanism of action of anti-CD47 antibodies:
One is that cancer cells up-regulate expression of CD47 to fool macrophages, while anti-CD47 antibodies enable macrophage to bring into play the phagocytosis by blocking "Don't eat me" signal. For this mechanism, Stanford University Dr. Weissman, Founder of Forty Seven, has conducted a series of work for demonstration, as shown in the following figure:
(Source: labweb.cn; biodiscover.com)
The other one is that anti-CD47 antibodies bring into play the tumor cytotoxicity through DCs and CD8+T. DCs produce synergistic effect with pro-phagocytic molecules via anti-CD47 antibodies, to swallow tumor cells, and present tumor-associated antigens to CD8+T, thus to bring into play the specific cytotoxicity of CD8+T on tumors. The research results were published on Nature Medicine online by the team led by Fu Yangxin, a member of Institute of Biophysics, Chinese Academy of Sciences and a professor at University of Chicago, as shown in the following figure:
(Source: Nature Medicine, Note: Anti-CD47 antibody① and pro-phagocytic cell molecule such as calreticulin② synergistically enhance APC③’s phagocytosis of tumors, to process and present tumor antigens to become the peptide④ in the groove of MHC class I molecule and start CD8+ T cell⑤ that has tumor antigen specificity)
Progress of Hengrui and Forty Seven separately ranking first in China and overseas
Most of the anti-CD47 antibodies currently in development include active Fc domains and allow the maximum effects of Fc-dependent functions like CDC/ADCC; IgG4 domain is considered to have complete effector function, while IgG1 only has partial activity. The anti-CD47 antibodies include monoclonal antibody, bispecific antibody and antibody fusion protein, etc. There have been anti-CD47 antibodies of 6 companies in the world that enter the clinical trials, as shown in the following table:
|
Pharmaceutical product
|
Company
|
Ingredient
|
Fc region
|
R&D stage
|
Trial start time
|
|
Hu5F9
|
Forty Seven
|
Humanized monoclonal antibody
|
IgG 4
|
Phase Ⅱ clinical trial
|
August 2014
|
|
CC90002
|
Inhibrix/Celgene
|
Humanized monoclonal antibody
|
IgG 4
|
Phase I clinical trial
|
March 2015
|
|
SRF-231
|
Surface Oncology
|
Humanized monoclonal antibody
|
IgG 4
|
Phase I clinical trial
|
March 2018
|
|
TTI-621/622
|
Trillium Therapeutics
|
SIRPα-Fc fusion protein
|
IgG 4/1
|
Phase I clinical trial
|
January 2016 / May 2018
|
|
ALX-148
|
Alexo
|
SIRPα-Fc fusion protein
|
Inactive Fc region
|
Phase I clinical trial
|
February 2017
|
|
SHR-1603
|
Hengrui
|
/
|
/
|
Approved for Phase I clinical trial
|
/
|
(Source: Official websites of companies)
Wherein, Forty Seven, Inc. has the most advanced clinical R&D pipeline, and has many projects at Phase Ib/II trial, followed by Trillium Therapeutics, while, Hengrui is the number one in terms of progress in China.
Extensive indications—a market opportunity worth billions of USD
Anti-CD47 antibodies have many indications, including non-Hodgkin lymphoma (NHL), acute myeloid leukemia (AML) and other hematological malignancies, as well as colorectal cancer, ovarian cancer, bladder cancer, head and neck cancer, and other solid tumors, standing for hundreds of thousands of new patients every year and a market opportunity that is worth billions of USD.
Anti-CD47 antibody therapies are mainly divided into three research directions at present: single drug, combination with therapeutic antibody drugs, and combination with T cells and checkpoint inhibitors, wherein, Forty Seven’s safety and effectiveness study of Hu5F9-G4 in combination with rituximab to treat relapsed/refractory NHL has entered Phase Ib/II clinical trial, with relevant data published at the ASCO Annual Meeting this year: (n=22), ORR was 50%, and CR was 36%. Responses to those data are relatively positive, as the enrolled patients failed the treatment with multiple lines of drugs.
Clinical trial application of Innovent Biologics has been accepted by CDE
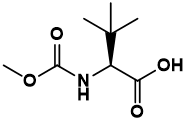
It's worth mentioning that, in China, Innovent Biologics is also actively acting, besides Hengrui that keeps up with the international pace. As far as I know, the product with code of IDI188 in the R&D pipeline of Innovent Biologics is an anti-CD47 antibody which has been applied for clinical trial and officially accepted by CDE on July 4, 2018.
|
Acceptance No.
|
Pharmaceutical product name
|
Registration classification
|
Application type
|
Acceptance date
|
Enterprise name
|
Handling status
|
Status start date
|
Task type
|
Serial No.
|
Review conclusion
|
View details
|
|
CXSL1800074
|
IBI188
|
Biological product for treatment 1
|
New drug
|
July 4, 2018
|
Innovent Biologics, Inc.
|
Under review
|
July 2, 2018
|
Clinical trial application
|
View
|
View
|
View
|
(Source: db.yaozh.com)
-----------------------------------------------------------------------------------------------------------------------------
Editor's Note:
To apply for becoming a contributor of en-CPhI.cn,
welcome to send your CV and sample works to us,
Email: Julia.Zhang@ubmsinoexpo.com.





 ALL
ALL Pharma in China
Pharma in China Pharma Experts
Pharma Experts Market News
Market News Products Guide
Products Guide Brand Story
Brand Story



















 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025








