Merck & Co.'s effort to take its Fosamax appeal to the Supreme Court just got a boost, and the entire pharma industry should take note. The U.S. Solicitor General is urging the justices to take on the appeal, which centers on a set of closely watched lawsuits alleging Merck failed to fully flag the risks of its osteoporosis drug Fosamax.
If the high court hears the drugmaker’s appeal, its decision could ripple through product liability lawsuits across the drug business. That's because at its heart is the issue of preemption: whether FDA decisions can protect drugmakers from state-level legal challenges.
Last year, a federal appeals court in New Jersey revived the previously dismissed Fosamax litigation and allowed a jury to examine whether Merck was entitled to protection, based on the FDA's decision-making on the drug's official label. In 2009, the agency had rejected Merck’s request to update Fosamax’s label with certain fracture risks, and Merck now argues that the FDA's decision precludes state tort claims.
After the Supreme Court asked the Solicitor General to weigh in, the nation's top lawyer sided with Merck, saying that preemption should be determined by a judge, not a jury, according to a brief (PDF).
"Where, as here, FDA renders a decision declining to approve a drug-labeling change, the interpretation of that administrative decision and its significance for a failure-to-warn claim are legal questions for a court to resolve, not factual questions for a jury," wrote the Solicitor General.
"Judges, rather than lay juries, are best suited to evaluate the scope of an agency’s legal determination in light of the relevant statutory and regulatory context," the Solicitor General added.
More than 500 patients who took Fosamax suffered femoral fractures before the FDA added a warning of that risk to its label in 2011.
Merck previously argued that preemption should apply because it had attempted to update the label earlier, only to be defeated by the FDA because of wording. But the patients contended that the "stress fracture" term used in Merck’s request did not describe the injuries they experienced. "[Merck] proposed a warning only about a different risk," therefore, the FDA never rejected a warning of "the risk at issue," the attorney representing the patients previously said.
In his brief—and in a victory for Merck—the Solicitor General said the FDA’s rejection of the label change was "clear evidence" supporting preemption. "[B]ecause FDA’s decision here prevented [Merck] from modifying the relevant labeling before late 2010, the court of appeals erred in rejecting [Merck’s defense]."
Plus, the Solicitor General argues that the FDA’s decision was actually based on the lack of adequate data to support a warning. "If a warning is warranted, FDA will attempt promptly to identify easily correctable deficiencies in the proposed text and will then develop final labeling text with the manufacturer in an iterative process."
Now, the Supreme Court will decide whether to review Merck’s case. If it takes the suit, it could become another landmark preemption case after Wyeth v. Levine, where the Supreme Court opens up the possibility of lawsuits against drugmakers even if a medication wins FDA approval.
Register as Visitor to CPhI China 2018!





 ALL
ALL Pharma in China
Pharma in China Pharma Experts
Pharma Experts Market News
Market News Products Guide
Products Guide Brand Story
Brand Story

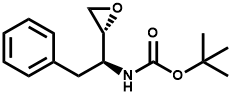
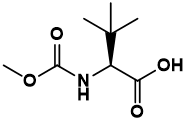


















 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025








