Kaléo announced the U.S. Food and Drug Administration (FDA) has approved its supplemental New Drug Application (sNDA) for AUVI-Q (epinephrine injection, USP) 0.1 mg, the first and only epinephrine auto-injector (EAI) specifically designed for the treatment of life-threatening allergic reactions, including anaphylaxis, in infants and small children weighing 16.5 to 33 pounds (7.5 to 15 kilograms) who are at risk for or have a history of serious allergic reactions.
The sNDA for the AUVI-Q 0.1 mg Auto-injector was granted Priority Review by the FDA, an expedited regulatory pathway reserved for products that may provide significant improvements in the safety or effectiveness of the treatment, diagnosis, or prevention of serious conditions when compared to available therapies.
AUVI-Q is a compact epinephrine auto-injector with industry-first features, including a voice prompt system that guides a user with step-by-step instructions through the delivery process, and a needle that automatically retracts following administration. The new 0.1 mg-dose epinephrine auto-injector has a shorter needle length and lower dose of epinephrine than current FDA approved 0.15 mg and 0.3 mg epinephrine auto-injectors.
"Today's decision by the FDA to approve the AUVI-Q 0.1 mg Auto-injector is exciting for all of us in the life-threatening allergy community who have been working for many years to fulfill this unmet medical need," said Spencer Williamson, President and CEO of kaléo. "As a company that focuses on patients first, and providing potentially life-saving treatments, we are particularly glad we will be able to help caregivers by providing an EAI that was specifically designed with an appropriate dose and needle length for infants and children (16.5 to 33 pounds) in order to maximize the potential for a safe administration of epinephrine."
AUVI-Q 0.1 mg has a dose and needle length designed specifically for treating anaphylaxis in infants and small children weighing 16.5 - 33 pounds. AUVI-Q 0.1 mg includes the innovative AUVI-Q electronic voice instruction system as well as visual cues to help guide users step-by-step through the administration.
The goal of inventors of AUVI-Q was to develop an epinephrine auto-injector that contained innovative features, such as a voice instruction system that helps guide patients and caregivers step-by-step through the injection process.
The AUVI-Q 0.1 mg Auto-injector is projected to be available for patients in the first half of 2018.





 ALL
ALL Pharma in China
Pharma in China Pharma Experts
Pharma Experts Market News
Market News Products Guide
Products Guide Brand Story
Brand Story
![N-(8-[2-hydroxybenzoyl] amino) caprylate (SNAc) - Salcaprozate sodium](https://eimg.pharmasources.com/image/20250407/glWQx8r7zi06C4pGLlnVnwh4wzxTuy5ctYzly6p1.png)
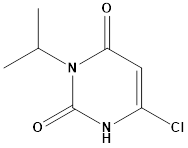
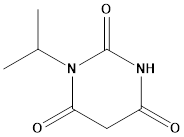
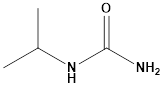
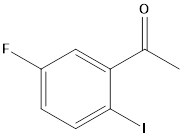


























 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025








