According to reports, GlaxoSmithKline's (GSK) shingles vaccine candidate, Shingrix was unanimously recommended for approval by the U.S. Food and Drug Administration's (FDA) Vaccines and Related Biological Products Advisory Committee. A U.S. Food and Drug Administration advisory panel on Wednesday voted 11-0 for the vaccine for its safety and efficacy.
A Reuters report mentioned that Panel members were "very impressed" by efficacy data from Shingrix clinical trials, and that it represents an improvement over Zostavax, the only marketed shingles prevention vaccine from Merck & Co. GSK's shingles vaccine candidate is not currently approved for use anywhere in the world. Regulatory filings in the European Union, Canada, Australia and Japan are underway.
The Biologics License Application (BLA) was submitted to the FDA in October 2016. The FDA will consider the Advisory Committee vote as it reviews the BLA, although it is not required to follow the recommendation.
Dr. Emmanuel Hanon, Senior Vice President and Head of Vaccines R&D for GSK said: "Shingles is a painful and potentially serious condition. The risk of developing shingles increases with age and it is estimated that up to one in three people in the United States will develop shingles. Today's vote brings us one step closer to approval of Shingrix, which is specifically designed to overcome age-related weakening of the immune system."
Shingrix, contains Agenus' proprietary immune adjuvant, QS-21 Stimulon. Agenus is a clinical-stage immuno-oncology company focused on the discovery and development of therapies that engage the body's immune system to fight cancer. "The Advisory Committee's recommendation for the approval of Shingrix marks the first for a product that includes Agenus' proprietary immune adjuvant, QS-21 Stimulon, and serves as a significant validation," said Garo Armen, Ph.D., Agenus CEO and Chairman of the Board. "In addition to being studied in diverse development stage vaccines, QS-21 Stimulon is also a critical component of our neoantigen vaccine formulations. We believe QS-21 provides Agenus with a competitive advantage due to its demonstrated ability to bolster immunogenicity in diverse vaccine formulations offering potential benefit to patients."
GSK said in June that the vaccine produced a strong immune response in adults 65 and older who had previously been vaccinated against shingles with Merck’s vaccine, Zostavax. Scientific data published in the New England Journal of Medicine showed that the effectiveness of Merck’s vaccine wanes over time, while GSK’s vaccine appeared to have longer-lasting protection.
GSK said data show that people who received Merck’s vaccine, the only one approved now for the herpes zoster (shingles) vaccine, can later receive the Shingrix vaccine safely and effectively.
Shingles is a painful skin rash camera.gif. It is caused by the varicella zoster virus. Shingles usually appears in a band, a strip, or a small area on one side of the face or body. It is also called herpes zoster. Shingles is most common in older adults and people who have weak immune systems.





 ALL
ALL Pharma in China
Pharma in China Pharma Experts
Pharma Experts Market News
Market News Products Guide
Products Guide Brand Story
Brand Story
![N-(8-[2-hydroxybenzoyl] amino) caprylate (SNAc) - Salcaprozate sodium](https://eimg.pharmasources.com/image/20250407/glWQx8r7zi06C4pGLlnVnwh4wzxTuy5ctYzly6p1.png)
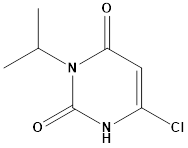
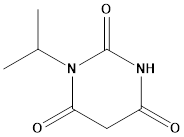
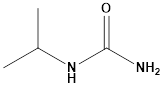
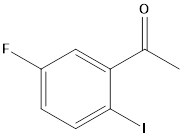





















 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025








