Bristol-Myers Squibb and Apexigen are to test a combination of Opdivo and the latter’s investigational compound APX005M in patients with advanced solid tumours.
The proposed collaboration will look at the safety, tolerability and preliminary efficacy of the combination in second-line metastatic non-small lung cancer patients who have failed prior chemotherapy, and in metastatic melanoma patients who have failed prior immuno-oncology therapy.
Preclinical data incidate that APX005M mimics the endogenous immune activation process through activation of CD40, a receptor on the surface of antigen presenting cells of the immune system which plays a fundamental role in the activation of both innate and adaptive immune system mechanisms.
Opdivo was the first PD-1 immune checkpoint inhibitor to receive the first global regulatory approval in July 2014, and has since racked up approval in 57 countries including the US, Japan, and in the EU.
The companies will assess the potential of combining these two agents with the goal of effectively activating antigen presenting cells (APC) in the tumour microenvironment to drive a more productive and sustained immune response.
"Targeting the tumor microenvironment through activation of antigen-presenting cells is a novel approach that we are excited to add to our Immuno-Oncology strategy as we continue to advance research for cancers with limited treatment options," stated Fouad Namouni, head of Oncology Development at BMS.
"Our agreement with Apexigen builds on our continued focus to bring forward potential novel combination treatment options for patients with cancer."





 ALL
ALL Pharma in China
Pharma in China Pharma Experts
Pharma Experts Market News
Market News Products Guide
Products Guide Brand Story
Brand Story


















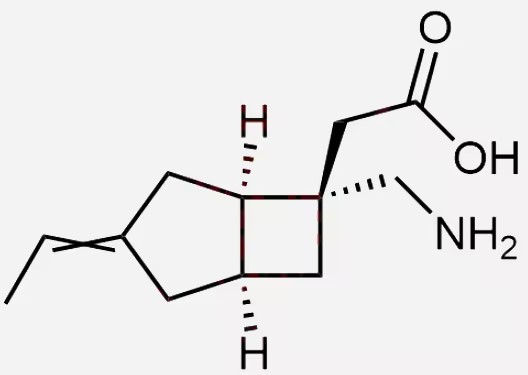


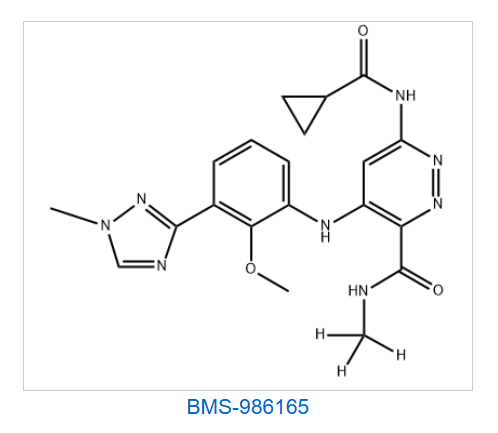
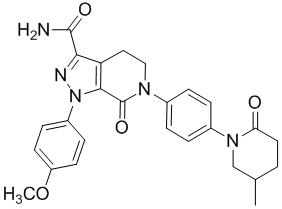







 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025








