January 07, 2025
Tag:
On December 30, 2024, hydroxypropyl chitosan, a pharmaceutical excipient produced by Shandong Binzhou Zhiyuan Biotechnology, successfully completed the filing review of the China Food and Drug Administration (CDE). CDE registration number: F20240000717.
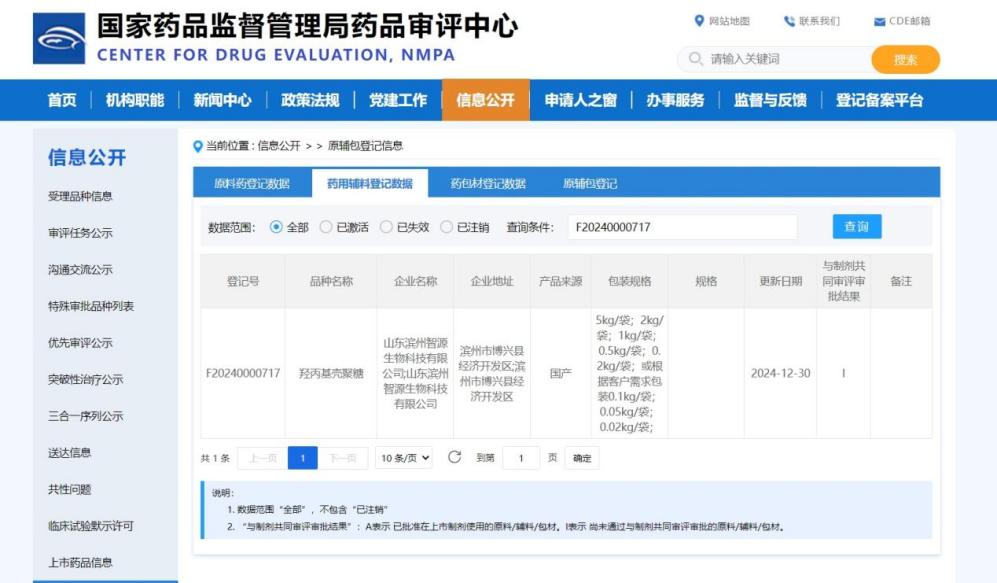
The completion of the CDE filing marks that the hydroxypropyl chitosan produced by Zhiyuan Biotechnology meets the domestic standards for pharmaceutical excipients in terms of quality and safety, making the company stand out in the pharmaceutical excipients market, gain more trust and cooperation opportunities from pharmaceutical companies, expand market share, and enhance the visibility and influence of the company in the industry.


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
Follow Us:




 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025


