March 28, 2023
Tag:
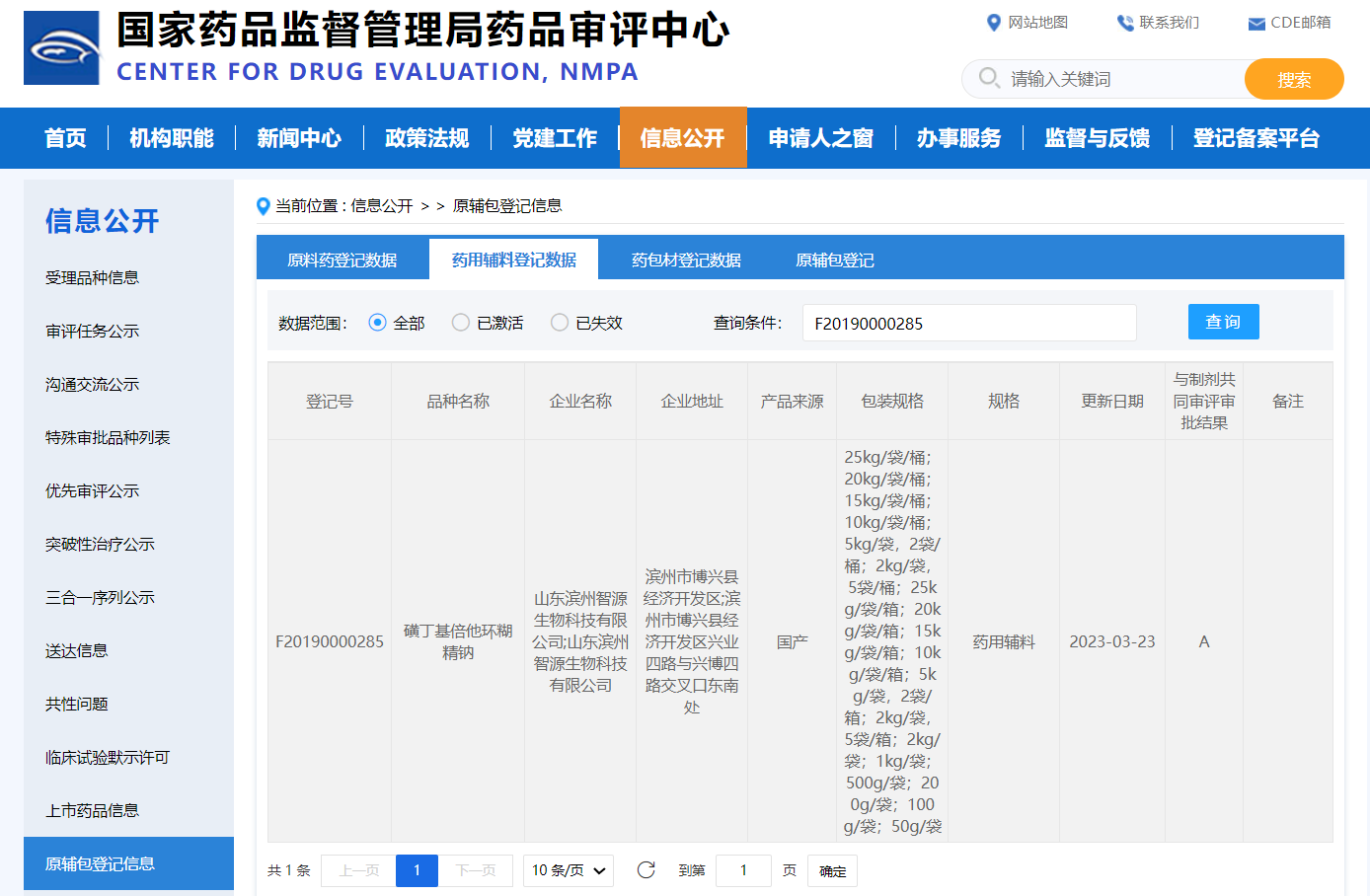
The CDE registration number of the pharmaceutical excipient Betadex sulfobutyl ether sodium developed by Shandong Binzhou Zhiyuan Biotechnology Co., Ltd. was successfully transferred to "A" on March 23, 2023!
This is a major breakthrough in the field of pharmaceutical excipients in my country, and it is also the result of Zhiyuan Biotechnology's unremitting efforts over the years. Betadex sulfobutyl ether sodiumis a new type of excipient for pharmaceutical preparations, which can form inclusion complexes with drug molecules to improve the water solubility, stability and bioavailability of drugs. Currently, it is mainly used for voriconazole and posaconazole Solubilize drugs such as azoles, carfilzomib, remdesivir, etc. The Betadex sulfobutyl ether sodium of Zhiyuan Biotechnology is a high-quality product with independent intellectual property rights of high purity, low nephrotoxicity, low hemolysis, low endotoxin and low metal ion content! Since this product is an injection-grade excipient and its dosage in the preparation is dozens of times that of the API, the hardware and software requirements for production management have reached the extreme. During the new crown epidemic, this product is the key to the anti-new crown drug remdesivir Excipients have successfully saved tens of millions of lives.
This project is a national key research and development project of the Ministry of Science and Technology "Technology Boosts Economy 2020" undertaken by our company. This is also the first national-level scientific research project independently undertaken by an enterprise in our county in more than ten years.
As a national-level "specialized, special and new" key little giant, Zhiyuan Biotechnology has a high-quality, professional and international R&D team. We cooperate with Simcere Pharmaceuticals to jointly promote the development and innovation of new drugs in my country. Over the past 13 years, a total of 886 person-times of online meetings have been held, 238 person-times of the two parties have been on-site for exchanges, more than 2,000 patents and literature have been consulted, and hundreds of text materials have been formed. For 4D, the two parties invested a total of more than 80 million yuan in software and hardware construction, and finally achieved a satisfactory result!
As a national high-tech enterprise, Zhiyuan Biotechnology always adheres to customer-centricity, innovation as the driving force, quality as the life, and is committed to providing customers with high-quality pharmaceutical raw materials and services. Transfer of excipients to "A" means that after the association review of excipients and preparations is passed, the excipients obtain the drug market access qualification and enter the domestic market legally and compliantly for sale. We sincerely invite pharmaceutical companies, experts and scholars to contact us to discuss the application prospect and potential of Betadex sulfobutyl ether sodium in various pharmaceutical preparations. We believe that through cooperation, we can make more contributions to the cause of human health.
Special thanks to our customers for their recognition, trust and recommendation!
Special thanks for the policy support given by the Third Sub-bureau of Regional Inspection of Shandong Drug Administration!
Special thanks to the following companies and teams:
Simcere Pharmaceuticals
Hao Aiyou team of Shandong University;
Nankai University Liu Yu team;
The team of Tu Jiasheng and Sun Chunmeng, China Pharmaceutical University;
Professor Sun Tao, School of Pharmacy, Fudan University;
Thank you for your technical support!
Thank you for our unremitting efforts to achieve the most satisfactory results!


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
Follow Us:




 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025


