May 07, 2022
Tag:
Sodium Hyaluronate (also known as Hyaluronic Acid) is a linear polymer acidic monosaccharide formed by d-glucuronic acid (GlcA) and N-acetylglucosamine (GlcNAc), which are connected alternately in disaccharide units. Sodium hyaluronate widely exists in the body. Sodium hyaluronate has many physiological functions, such as regulating adhesion, localization and differentiation of cell to cell, promoting cell proliferation and movement, and taking part in biological processes such as moisturizing, lubricating, wound healing, tissue repair and regeneration, inflammatory response, embryonic development and tumor. At present, the application of Sodium Hyaluronate is mainly concentrated in medicine, clinical diagnosis and treatment, cosmetics and health food industry because of its product characteristics. In the medical field, it is not only widely used in various kinds of ophthalmic surgery, such as lens implantation, corneal transplantation and anti-glaucoma surgery, but also can be used to treat arthritis and accelerate wound healing.
When Sodium Hyaluronate is used for medicinal or medical purposes, bacterial endotoxins as an important indicator has always been the focus of relevant personnel in the analysis and detection reports, and this index will be controlled in the production process also.
What is bacterial endotoxins?
Bacterial endotoxins, also known as lipopolysaccharide, is the main structure of the outer membrane of Gram-negative bacteria. It will fall off from the outer membrane of bacteria and be released into the surrounding media during the various stages of bacterial growth and reproduction as well as when the bacteria are autolyzed or lysed.
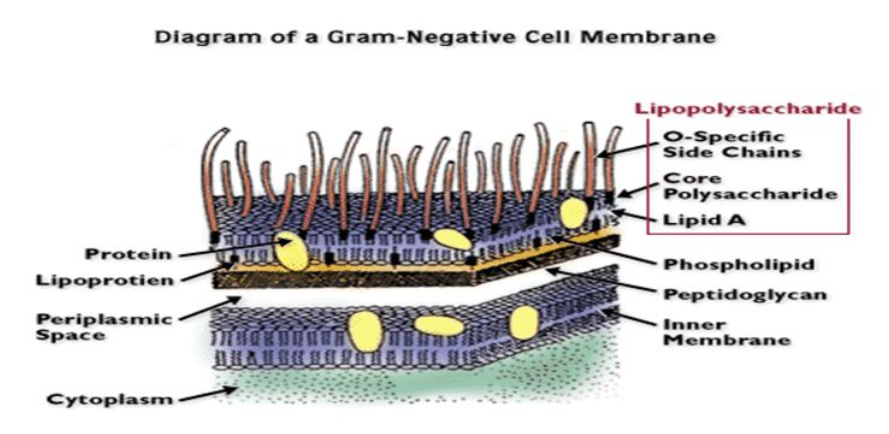
Endotoxins can cause intravascular blood coagulation, which is caused by the large amount of tissue factor induced by endotoxins in monocytes and vascular endothelial cells. In severe trauma, infection and sepsis, the body has hypermetabolic reactions such as body temperature rise, weight loss and energy loss. The main pathogenic mechanism is that bacterial endotoxins activates a variety of cells in the body to produce and release cytokines to participate in the body's acute Period response.
Bacteria grow and multiply at the site of infection, releasing endotoxins into the blood may cause diffuse intravascular coagulation, acute lung injury and acute respiratory distress syndrome, liver and bile injury, endotoxins shock and other hazards. Moreover, due to the use of antibacterial drugs, a large number of bacteria are killed, resulting in the release of endotoxins and aggravation of the disease. Therefore, bacterial endotoxins is a key parameter that needs to be controlled.
The bacterial endotoxins of Sodium Hyaluronate mainly comes from gram-negative bacteria, which may be introduced from the materials used and the environment during production process. The bacterial endotoxins is produced when gram-negative bacteria are autolyzed or lysed.
In recent years, the distribution, chemical structure, physiological function and clinical application of Sodium Hyaluronate have been studied in detail and deeply. Sodium Hyaluronate has been widely used in Ophthalmic Viscosurgical Device (OVD), Intra-articular Injection, soft tissue repair, and anti-adhesion agent after surgery. In order to ensure the safety of clinical use, bacterial endotoxins-induced pyrogen is also one of the important quality control items.
According to national drug standards, the value of bacterial endotoxins contained in Sodium Hyaluronate per 1 mg should be less than 0.5 EU for ophthalmic injection and less than 0.05 EU for orthopedic injection.
Bacterial endotoxins (2.6.14): The Description of Bacterial endotoxins (2.6.14) in European Pharmacopoeia is as follows.
Less than 0.5 IU/mg, if intended for use in the manufacture of parenteral preparations without a further appropriate procedure for the removal of bacterial endotoxins;
Less than 0.05 IU/mg, if intended for use in the manufacture of intra-ocular preparations or intra-articular preparations without a further appropriate procedure for the removal of bacterial endotoxins.
Bacterial endotoxins level of Topscience
In view of the importance of controlling bacterial endotoxins, Topscience has been working on lower levels of bacterial endotoxins. According to the bacterial endotoxins test results of Sodium Hyaluronate in successive batches, the bacterial endotoxins level of Topscience is around0.001 IU/mg
Topscience has now upgraded the in-house quality standard of Sodium Hyaluronate injection grade, and the upper limit of bacterial endotoxins has been updated to 0.005 IU/mg, which confirms the high-quality assurance of Topscience.
At present, the level of bacterial endotoxins in the industry is generally controlled at < 0.05 IU/mg according to the pharmacopoeia standard. the bacterial endotoxins level of Topscience is around 0.001 IU/mg, which was 50 times lower than the pharmacopoeia standard. For bacterial endotoxins of Sodium Hyaluronate, Topscience is in the leading position in the industry.
Prospects of Topscience from the perspective of bacterial endotoxins
The level of bacterial endotoxin of Topscience is 50 times lower than the standard of pharmacopoeia, which is due to the strict and efficient control of the product quality from the collection of raw materials to the release of products. Topscience’s Sodium Hyaluronate is produced by microbial fermentation, the raw material is not from animal, and the impurity level of the product is low, including bacterial endotoxin, heavy metal, elemental impurity, microbial contamination, etc., which all are controlled at the lowest level in the industry.


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
Follow Us:




 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025


