November 26, 2024
Tag:
With the prevalence and outbreak of various viruses, people need more research, development, and production of human and veterinary viral vaccines.
High-yield vaccine production requires large-scale cell culture, which necessitates a supporting surface for adherent cells to attach to. Cells rely on adhesion factors secreted by themselves or provided in the culture medium to grow and reproduce on this surface.
Adherent cells pose a challenge for scale-up, and traditional methods such as roller bottles and cell factories require high consumable usage, large floor space, and high culturing costs. However, with the advancement of new technologies, microcarrier technology has become increasingly mature, and its application in single-use reactors is now also relatively well-established. There is a growing trend towards selecting single-use reactors for new drug production lines.
Single-use reactors offer higher controllability, efficiency, and flexibility. They eliminate the need for cleaning validation required by traditional stainless-steel bioreactors. As upstream technologies such as perfusion continue to improve, the production of biopharmaceuticals is gradually shifting towards smaller upstream culture scales and larger downstream purification scales. The application of single-use technologies in perfusion-related processes is also becoming more intelligent.
Microcarriers are used to produce the vast majority of attenuated or inactivated vaccine types for human and veterinary use in many countries around the world.
Vaccines produced using microcarriers include:
Poliovirus vaccine, Measles virus vaccine, Rabies virus vaccine, Influenza virus vaccine, Japanese encephalitis virus (JEV) vaccine, Zaire Ebola virus (ZEBOV) vaccine, Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV2) vaccine, and Foot-and-Mouth Disease Virus (FMDV) vaccine.
Additionally, there are many viral vaccines in research and development that utilize microcarrier production, such as Chikungunya virus vaccine, Dengue fever virus vaccine, Hand-Foot-and-Mouth Disease (EV71) vaccine, Hantaan virus vaccine, West Nile virus vaccine, Respiratory Syncytial Virus (RSV) vaccine, and Yellow fever virus vaccine. Compared to other cell culture processes, microcarrier culture technology can improve yield, reduce costs, and minimize contamination [1].
In addition, microcarrier culture technology is also widely used in the development of veterinary vaccines:
Such as Classical Swine Fever Virus (CSFV), Pseudorabies Virus (PRV), Porcine Parvovirus (PPV), Canine Distemper Virus (CDV), Porcine Epidemic Diarrhea Virus (PEDV), and Porcine Circovirus 2 (PCV2) viral vaccines.
Selection Methods for Microcarriers
Spherical microcarriers refer to microspheres with a diameter ranging from 60 to 250 μm, which are suitable for the growth of adherent cells. Since Van Wezel et al. developed the first microcarrier using DEAE-Sephadex A-50 in 1967, after more than half a century of application and optimization in the market, microcarriers have been widely used in various biomedical fields such as vaccines, monoclonal antibodies, recombinant proteins, cell therapy, stem cell culture, primary cell culture and expansion, cellular agriculture, tissue regeneration, and drug delivery.
Key Parameters of Spherical Microcarriers [2]
1. Surface Charge:
Typically, microcarriers carry a positive charge, and the charge density is a critical factor affecting cell growth. The charge density of microcarriers should be controlled within the range of 1-2 meq/g. If it is lower than 1 meq/g, it may lead to insufficient or no cell attachment. However, excessively high charge density can cause "toxic" effects or inhibit cell growth.
2. Diameter:
The diameter of microcarriers is generally not less than 50-70 μm (not nm as originally stated, as 50-70 nm would be too small for cell growth). A diameter too small can inhibit cell growth on the microcarriers. Typically, a diameter range of 100-250 μm is suitable for microcarriers. Additionally, minimizing the variation in the uniformity of microcarrier diameters is important to ensure consistency in the timing and density of cell harvest.
3. Density:
The density of microcarriers should be slightly greater than that of the culture medium to facilitate separation from the medium, but not too high to ensure that they can remain suspended at low rotation speeds, thus avoiding cell damage caused by shear forces generated at high rotation speeds. Therefore, the density of microcarriers is generally within the range of 1.03-1.05 g/ml.
4. Inoculation Density:
The cell inoculation density is closely related to factors such as cell type, microcarrier dosage, and culture conditions. Typically, the microcarrier dosage ranges from 3-20 g/L. Depending on the cell type and culture conditions, the inoculation density per microsphere can be 10-50 cells, and the final cell expansion can be 3-10 times.
Currently, microcarrier culture technology is primarily applied in the production of viral vaccines, with many types of viral vaccine production reaching scales of 1000-6000 liters. Additionally, there are numerous novel viral vaccines under research that utilize microcarrier technology for scale-up cultivation.
The application of microcarrier culture technology in the field of viral vaccines benefits from the development and utilization of Vero cells. The Vero cell line is the first continuous cell line (CCL) approved by the WHO for the production of human viral vaccines. Vero cells have been used as a substrate for human vaccines for over 30 years, with hundreds of millions of doses of vaccines produced and sold globally [1]. Since Vero cells are interferon-expression-deficient and have a weak innate immune response, they are susceptible to various viruses and can easily produce high titers of viruses.
Therefore, the application of Vero cells in the viral vaccine industry holds an equally important position as CHO cells in the antibody field. Additionally, microcarriers are also suitable for the growth of various other adherent cells, such as MDCK, MARC-145, BHK-21, and PK-15, which play crucial roles in the production of various types of viral vaccines.
To meet the market demand for microcarriers, BioLink has launched Puredex® Cyto-1 microcarriers.
Puredex® Cyto-1 is a type of microcarrier based on a cross-linked dextran scaffold with positively charged groups. It provides a vast attachment surface for cells, thereby increasing cell density and achieving high yields. Puredex® Cyto-1 microcarriers not only make it possible to change the culture method for adherent cells but also facilitate the optimization of cell culture processes, leading to cost reduction and efficiency improvement. Puredex® Cyto-1 microcarriers can be used for the culture of various host cells.
|
Name |
Description |
|
Matrix |
Cross-linked dextran |
|
Ligand |
N-N-Diethylaminoethyl |
|
Average particle size |
190 μm |
|
Specific surface area |
4000 cm2/g dry weight |
|
Number of microspheres |
4X106/g dry weight |
|
Swelling ratio |
20ml/g dry weight |
Parameter Table of Puredex® Cyto-1 Microcarriers
The experimental results of using BioLink's Puredex® Cyto-1 microcarriers in the CytoLinX® BR Single-use Bioreactor are as follows:
Adherence of Vero Cells:
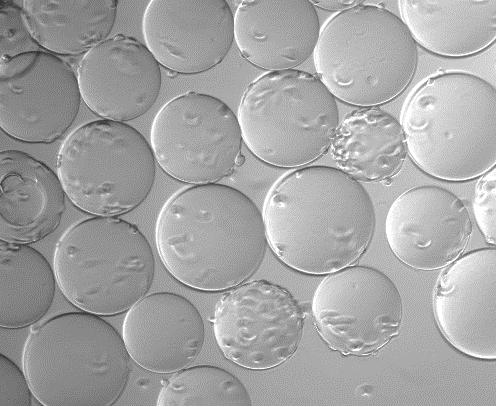
Adherence of Vero Cells After 2 Hours
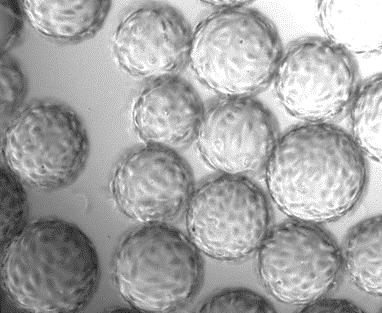
Adherence of Vero Cells After 24 Hours
Enabling Scale-up Production of Vero Cells in the Bioreactors
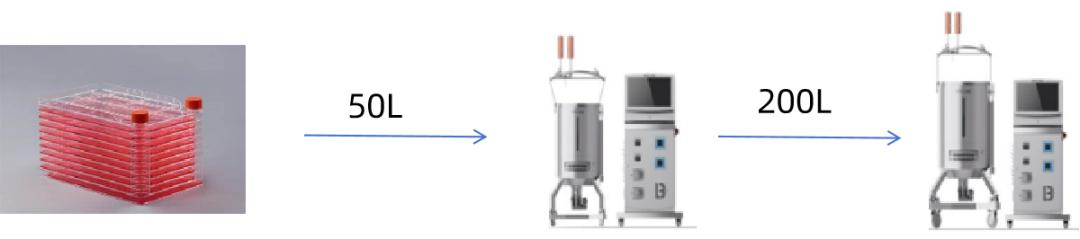
CytoLinX® BR Single-use Bioreactors Selection
The standard products of CytoLinX® BR Single-use Bioreactors are available with 50L, 200L, 500L, 1000L, and 2000L volumes. They are integrated with tanks, controllers, and temperature control units (TCU) and can be applied to industrial-scale mammalian and insect cell cultures as well as other cell cultures requiring low shear force.
Developed based on the DCS architecture, its underlying logic is built using Siemens PCS 7, covering the automation needs from research and development to production, and from a single machine to the entire upstream plant. Its automation logic is compliant with the ISA 88 control standard.
The software design meets the requirements of GMP and 21 CFR Part 11. At the same time, to facilitate user experience, the CytoLinX® BR software interface is designed with minimalist aesthetics, where all functions are clear at a glance and can satisfy customers' needs to a great extent.
The automatic control system satisfies functions such as real-time data recording, PID automatic control, online monitoring, and multi-parameter cascade control, and it exhibits stable pH, DO, and temperature control performance.
To achieve more efficient mixing, BioLink has introduced a brand-new bottom design that can maintain a high linear KLa. It also provides microbubble, medium bubble, macro bubble, and various combination consumable solutions to meet the demanding culture needs of various cells.

References:
1. Kiesslich, S. and A.A. Kamen, Vero cell upstream bioprocess development for the production of viral vectors and vaccines. Biotechnology Advances, 2020. 44.
2. Merten, O.-W., Advances in cell culture: anchorage dependence. Philosophical Transactions of the Royal Society B: Biological Sciences, 2015. 370(1661).


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
Follow Us:




 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025


