December 30, 2020
Tag:
Background and synopsis
Albiglutide is a weekly injectable GLP-I receptor stimulant developed by GlaxoSmithKline.On April 15, 2014, the US FDA approved subcutaneous injections of albiglutide in combination with diet and exercise to improve glycaemic control in adult patients with type II diabetes.
Albiglutide has a molecular formula of c3232h5032n8640979s41 and a relative molecular mass of 72970, and GLP. 1 analogs composed of 2 sequences of 30 amino acids were fused to recombinant human serum albumin to form fusions.Resistance to hydrolysis by dipeptidyl peptidase (DPP-4) was achieved by replacement of amino acid 8 (alanine for glutamate) of the complete human GLP. 1 amino acid sequence, with the formed fusion protein functioning to extend the half-life.Patients with T2D generally have reduced levels of GLP-1 compared with normal individuals.Albiglutide has incretin glucose lowering effects, promoting Glucose dependent insulin secretion, inhibiting glucagon secretion, slowing gastric emission, and promoting satiety.Albiglutide, as an analog of long-term GLP. 1, can reduce blood glucose levels in fasting and after meals.The fusion protein formed increases the half-life of the drug, but the fused macromolecular protein may affect drug and GLP. 1 receptor interactions, and studies in mice suggest that albiglutide may attenuate the effects of gastric emission and satiety due to changes in the relative molecular mass.
Pharmacol res
1. Dosing studies.
The leaders of Matthews et al., who participated in a randomized double-blind parallel trial of 54 patients with type II diabetes randomized to weekly injections of albiglutide 9 mg, 16 mg, 32 mg, showed that fasting and postprandial glucose levels were related to the injected dose.The relationship between dose and efficacy was further confirmed in the study by seino et al., in which the doses of albiglutide were 15 mg / week, 30 mg / week, 50 mg / week, 100 mg / week, or placebo in randomized double-blind parallel controlled trials that enrolled Japanese patients with type II diabetes.After 29 days of treatment, all treatment groups achieved significant improvements from baseline in HbA1c (- 0.58%, 0.57%, - 0.63%, - 0.51% P < 0.03) with the exception of the 100 mg / week group, which also achieved significant improvements from baseline in HbA1c in the 50 mg / week, 100 mg / week treatment groups, and other groups, with minimal adverse effects in the 30 mg / week treatment group.Therefore, the recommended dosage of the tanzeum instructions is once a week (abdominal, leg, upper arm subcutaneous injections).After 6 to 8 weeks of treatment, the dose can be increased to 50 mg weekly for poor glycemic lowering.
2. Pharmacokinetics.
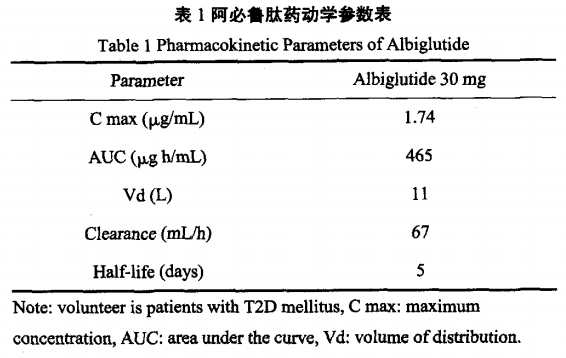
Through a clinical trial on 39 healthy volunteers, five different doses of albiglutide and placebo (cohoat1: 0.25 + 1 mg; cohort2: 3 + 6 mg; cohort3: 16 + 24 mg; cohort4: 48 + 60 mg; cohort5: 80 + 104 NAG) were randomly administered on days 1, 8.The results showed that the mean half-life (T1 / 2) was 6.8 days, the peak plasma concentration (Tmax) appeared on days 2.3-4, and there was a clear link between blood glucose concentration and dosage.In pharmacokinetic studies in patients with type II diabetes, the peak plasma concentration (Cmax) of 30 mg of albiglutide was 1.74The enzymes break down into peptides and single amino acids, and their metabolic pathways may be similar to those of human serum albumin, mainly through vascular endothelial breakdown.The study showed that the average apparent removal rate of this drug was 67 ml / h, and the removal half-life was approximately 5 days, and this drug was suitable for once a week.Detailed pharmacokinetic parameters are shown in Table 1.
3. Drug drug interactions.
In drug interaction studies, albiglutide was coadministered with digoxin, oral contraceptives, and warfarin without significant effects on their efficacy and metabolic rate.However, CO administration with simvastatin increased the Cmax of simvastatin and its active metabolite by 18% and 98%, decreased the area under the curve (AUC) of simvastatin by 40%, and increased the AUC of the active metabolite by 36%, leaving the clinical significance of these drug interactions unknown.
Safety study
1. Reproductive toxicity studies.
Currently, there is no evaluation of drug safety in pregnant women.Murine animal models have shown reproductive toxicity but no malformations when administered 39 fold the dose of albiglutide equivalent to 50 mg in humans, leading to the recommendation to discontinue the drug at least one month before a planned pregnancy.Albiglutide is a fusion protein formed in combination with serum albumin and may be present in the milk of humans, so discontinuation of lactation or discontinuation of the drug is recommended.
2. Pancreatitis, medullary thyroid tumor study.
Incretins, including albiglutide, are at risk for causing pancreatitis, 8 phase IIII trials in which patients with pancreatitis at presentation were 6 (6 / 2365, 0.3%) in the albiglutide group, 0 (0 / 468, 0%) in the placebo group, and 2 (2 / 2065, 0.1%) in the positive comparator group. 9 in the instructions for albiglutide, black box warnings are included that remind patients to respond to rodents and other GLP-1 analogs on the basis ofOf study, may cause medullary thyroid tumors.Therefore, the FDA requires that after the marketing of albiglutide, medullary thyroid tumors should be studied in relation to the drug for at least 15 years to evaluate its safety.
Write to the end:
GoToppeptide Biotech Co., Ltd. for a professional producer of peptides, and also to supply the drug substance of albiglutide, Hangzhou gotopbio is small famous in the field of domestic peptide company, the size of the company, although not very large, cattle not a lot, many difficult peptides, difficult modifications can be done.What's more, QC is rigorous and the price is reasonable.Companies or scientific institutes that need to buy antimicrobial peptides are welcome to purchase them.
IFor any requests of Peptide for research purpose, welcome to contact us. www.gtpeptide.com , sales1@gotopbio.com.


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
+86 15021993094
Follow Us:




 Pharma Sources Insight July 2025
Pharma Sources Insight July 2025


