February 28, 2022
Tag:
On February 16, 2022, the data on Paxlovid previously published by Pfizer and submitted to the FDA were published in the New England Journal of Medicine (NEJM).
This NEJM article published viral load data that Paxlovid reduced viral load by 0.868 log10 compared to controls for 5 days of treatment, which equates to a remarkable 7.38-fold reduction in viral load.
This pivotal study (NCT04960202) enrolled 2,246 patients with COVID-19, 1,120 received Paxlovid and 1,126 received placebo.
Interim analysis showed that 28 days after receiving treatment, Paxlovid reduced the risk of hospitalization and death by 89.1% (p<0.001).
Applying the final results of the ITT analysis, Paxlovid reduced the risk of hospitalization and death by 88.9% (p<0.001).
According to our summary, Paxlovid has the following four main features that bring it closer to being a "potent drug".
1. Paxlovid Significantly Reduces Hospitalization/Mortality in High-Risk Unvaccinated COVID-19 Patients
The final analysis of the EPIC-HR study showed that the proportion of COVID-19-related hospitalizations or all-cause deaths was reduced by 89% when Paxlovid was given within 3 days of symptom onset, and by 88% if treated within 5 days of symptom onset.
2. Paxlovid Reduced Viral Load by 7-Fold.
Analysis of the secondary endpoint of the EPIC-HR study showed that Paxlovid reduced viral load by approximately 7.38-fold after 5 days of treatment compared to the placebo group, demonstrating that Paxlovid has potent activity against COVID-19 and is the first antiviral agent to truly reduce viral load.
3. Paxlovid Also Reduces Hospitalization/Morbidity Death in Non-High-Risk Unvaccinated/High-Risk Vaccinated COVID-19 Patients
In an interim analysis of EPIC-SR, Paxlovid reduced hospitalization/mortality in non-high-risk unvaccinated/high-risk vaccinated COVID-19 patients by 70% compared to the placebo group; and no patients in the treatment group died of disease.
4. In Vitro Assays Showed that Paxlovid Effectively Inhibited the 3CL Protease of Omicron Mutant Strains, Suggesting that Paxlovid Has the Potential to Maintain Potent Antiviral Activity Against Omicron.
Omicron has few protease mutations, so this drug is also effective against mutant strains.
In addition to its extremely high efficacy, Paxlovid has been shown to be very safe.
In the adverse events observed for the Paxlovid clinical trial, the treatment and control groups experienced comparable adverse events, most of which were mild.
Caronic Anhydride, An Intermediate Of Paxlovid
Speaking of Caronic anhydride, let's first introduce Boceprevir.
In May 2011, the FDA approved the marketing of Merck's hepatitis C treatment, Victrelis, also known as Boceprevir. The structural formula is as follows.
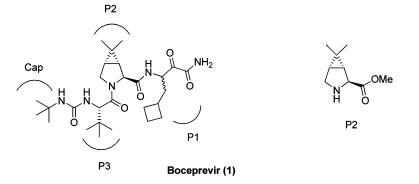
The molecular structure of Boceprevir consists of three fragments: P1, P2 and P3.
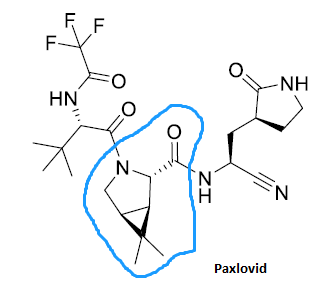
Caronic anhydride is the raw material for the synthesis of fragment P2. Here, fragment P2 can be referred to as the registered starting material and Caronic anhydride can be referred to as the raw material.
Pfizer's COVID-19 oral drug, Paxlovid, has a molecular structure that also includes fragment P2. With the growing popularity of Paxlovid, a second spring has arrived for caronic anhydride.
Huateng Pharma, one stop CDMO for pharma intermediates, can provide Paxlovid Intermediates with best price
▶ CAS NO.67911-21-1 | Caronic anhydride
▶ CAS NO.194421-56-2 | 6,6-Dimethyl-3-Azabicyclo[3.1.0]hexane-2,4-dione
▶ CAS NO.565456-77-1 | (1R,2S,5S)-Methyl 6,6-dimethyl-3-azabicyclo[3.1.0]hexane-2-carboxylate hydrochloride
▶ CAS NO.943516-54-9 | 6,6-Dimethyl-3-azabicyclo[3.1.0]hexane


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
Follow Us:




 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025


