October 14, 2020
Tag:
Peptides are a class of compounds composed of multiple amino acids linked by peptide bonds. They are ubiquitous in organisms. Up to now, tens of thousands of peptides have been found in organisms. Peptides play an important role in regulating the functional activities of various systems, organs, tissues and cells of the body, as well as in life activities, and are often used in functional analysis, antibody research and drug research and development. With the development of biotechnology and peptide synthesis technology, more and more peptide drugs have been developed and applied in clinic.
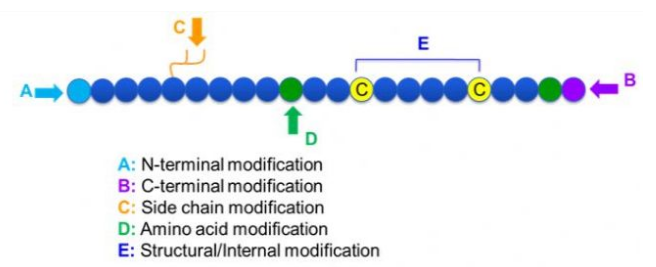
There are many kinds of peptide modification, which can be divided into post modification and process modification (using derivatized amino acid modification). According to different modification sites, it can be divided into N-terminal modification, C-terminal modification, side chain modification, amino acid modification, skeleton modification, etc. (Fig. 1). As an important method to modify the main chain structure or side chain group of peptide chain, peptide modification can effectively change the physical and chemical properties of peptide compounds, increase water solubility, prolong the action time in vivo, modify their biological dispersion, eliminate immunogenicity, and reduce toxic reactions. The main characteristics and the main modifications of peptides are introduced in this paper.
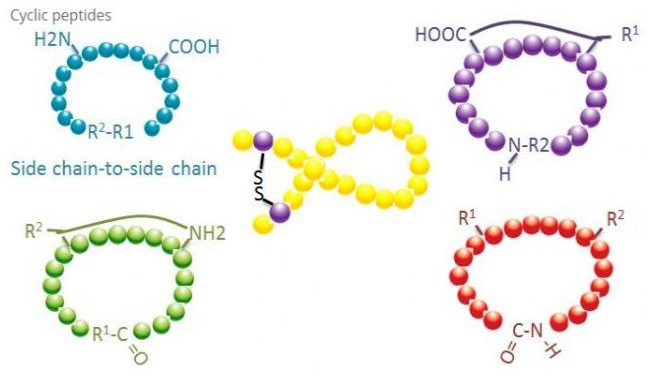
1. Cyclization
Cyclic peptides have many applications in biomedicine, and many bioactive natural peptides are cyclic peptides. Because cyclic peptides are often more rigid than linear peptides, they have strong resistance to the digestive system, can survive in the digestive tract, and show stronger affinity to target receptors. Cyclization is the most direct way to synthesize cyclic polypeptides, especially for peptides with large skeleton. According to the cyclization mode, it can be divided into side chain side chain type, terminal side chain type and terminal terminal terminal type (head and tail connected type).
(1) Side chain to side chain
The most common type of side chain side chain cyclization is disulfide bridge between cysteine residues. The method of introducing this cyclization is to form disulfide bond by oxidation of a pair of cysteine residues. By selectively removing the sulfhydryl maintenance group, the synthesis of polycyclic compounds can be completed. Cyclization can be accomplished in both the solvent after dissociation and the resin before dissociation. Because the polypeptides on the resin are not easy to form a cyclizable conformation, cyclization on resin may be less efficient than cyclization in solvent. Another variant of side chain side chain cyclization is to form amide structure between aspartic acid or glutamic acid residues and root amino acids, which requires that the side chain maintenance groups of polypeptides must be selectively removed either on the resin or after dissociation. The third side chain side chain cyclization is the formation of diphenyl ethers via tyrosine or p-hydroxyphenylglycine. The cyclization of this species in natural products is only found in microbial products, and the cyclization products often have potential pharmaceutical value. The preparation of these compounds requires common reaction conditions, so they are not often used in the synthesis of conventional peptides.
(2) Terminal to side chain
Terminal side chain cyclization usually involves the C-terminal amino group of lysine or ornithine side chain, or the N-terminal with aspartic acid or glutamic acid side chain. In addition, some polypeptides are cyclized by ether bonds formed by terminal C and serine or threonine side chains.
(3) Head to tail
The chain peptide can be cyclized in solvent or fixed on the resin through side chain cyclization. Low concentrations of polypeptides should be used for cyclization in solvents to prevent oligomerization of peptides. The yield of cyclic polypeptides is dependent on the sequence of chain polypeptides. Therefore, we should first create a possible chain like lead peptide library before preparing cyclic peptides in a large scale, and then stop cyclization to find the sequence that can achieve the best results.
2. N-methylation
N-methylation was initially present in natural peptides and was introduced into peptide synthesis to prevent the formation of hydrogen bonds, which made the peptides more resistant to biodegradation and elimination. In addition, N - (2-nitrobenzene sulfonyl chloride) peptide resin intermediate and methanol can be used to stop the Mitsunobu reaction. This method has been used to prepare cyclic peptide library containing N-methylated amino acid.
3. Phosphorylation
Phosphorylation is one of the most common post-translational modifications in nature. In human cells, more than 30% of proteins are phosphorylated. Phosphorylation, especially reversible phosphorylation, plays an important role in the control of many cellular processes, such as signal transduction, gene expression, cell cycle and cytoskeleton regulation, and apoptosis.
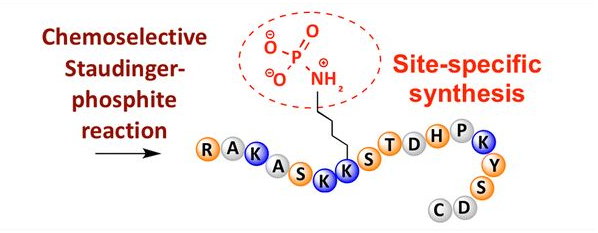
Phosphorylation can be observed on various amino acid residues, but the most common phosphorylation targets are serine, threonine and tyrosine residues. Phosphotyrosine, phosphothreonine and phosphoserine derivatives can be introduced into polypeptides or formed after peptide synthesis. Selective phosphorylation can be achieved by using serine, threonine and tyrosine residues which can selectively remove maintenance groups. Some phosphorylation reagents can also be modified to introduce phosphate groups into peptides. In recent years, the site-specific phosphorylation of lysine has been completed by using chemically selective Staudinger phosphite (Fig. 3)
4. Myristoylation and palmitoylation
Acylation of N-terminal with fatty acids can separate peptides or proteins from cell membranes. The N-terminal myristoylation sequence enables Src family protein kinases and reverse transcriptase gaQ proteins to target cell membrane separation. The N-terminal of the resin polypeptide can be conjugated with myristic acid by standard coupling reaction. The resulting lipopeptide can be dissociated under standard conditions and purified by RP-HPLC.
5. Glycosylation
Glycopeptides such as vancomycin and teicoplanin are important antibiotics for the treatment of drug-resistant bacterial infections. Other glycopeptides are often used to stimulate the immune system. In addition, because many microbial antigens are glycosylated, it is of great significance to study the therapeutic effect of glycopeptides on infection. On the other hand, it has been found that the proteins on tumor cell membrane show abnormal glycosylation, which makes glycopeptides play an important role in cancer and tumor immune defense research. The method of Fmoc / t-Bu was used to prepare glycopeptides. Glycosylated residues, such as threonine and serine, are often introduced into polypeptides by maintaining glycosylated amino acids through Fmoc activated by pentafluorophenol ester.
6. Isoprene
Isoprene is a cysteine residue in the left proximal chain of the C-terminal. Protein isoprene can improve cell membrane affinity and form protein protein interaction. The isoprene proteins include tyrosine phosphatase, small GTPase, chaperone, nuclear fiber layer and centromere isolate. Isoprene peptides can be prepared by isoprene on resin or by introducing cysteine derivatives.
7. Polyethylene glycol (PEG) modification
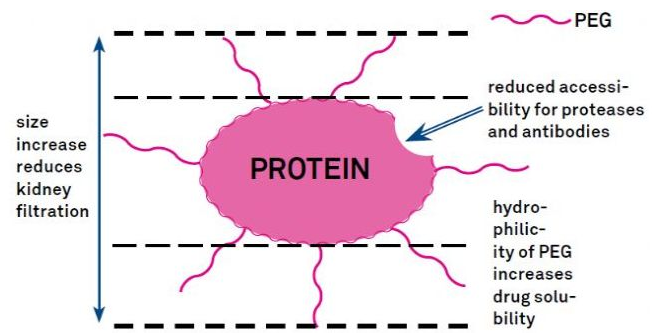
PEG modification can be used to improve the stability of protein hydrolysis, biological dispersion and peptide solubility. The introduction of PEG chains into polypeptides can improve their pharmacological properties and inhibit the hydrolysis of peptides by proteolytic enzymes. Peg peptides are easier to pass through the glomerular capillary cross-section than common peptides, which greatly reduces the renal clearance rate. Since the effective half-life of PEG peptide in vivo is prolonged, the use of peptide drugs with lower dose and lower frequency can maintain the normal treatment level. But PEG modification also has negative effect. A large amount of PEG can not only prevent the degradation of peptides by enzymes, but also reduce the separation of peptides and target receptors. However, the low affinity of PEG peptides is usually offset by its longer pharmacokinetics half-life. After being in the body for a longer time, PEG peptides are more likely to be absorbed by the target tissues. Therefore, the specification of PEG polymer should be optimized for the best results. On the other hand, PEG polypeptides accumulate in the liver to form macromolecular syndrome due to the decrease of renal clearance rate. Therefore, when peptides are used in drug testing, PEG modification should be designed more carefully.
The common modification groups of PEG modifiers can be summarized as follows: amino (- amine) - NH2, aminomethyl-ch2-nh2, hydroxy-oh, carboxyl-cooh, thiol (- thiol) - SH, maleimide mal, succinimide carbonate SC, succinimide acetate SCM, succinimide propionate spa, n-hydroxysuccinimide-nhs, propionyl-ch2ch2cooh, aldehyde CHO (such as propionaldehyde ALD, Butyrald, acrylate ACRL, azido azide, biotinyl biotin, fluorescein fluorescein, glutaric acid GA, hydrazide hydrazide, alkyne alkyne, p-toluenesulfonate OTS, succinimide succinate SS, etc. PEG derivatives with carboxylic acid can stop coupling with N-terminal amine or lysine side chain. Amino activated peg can be coupled with aspartic acid or glutamic acid side chain. Peg activated by mal can stop coupling with mercaptans of intact cysteine side chain [11]. The common classifications of PEG modifiers are as follows (Note: MPEG is methoxy peg, CH3O - (CH2CH2O) n-ch2ch2-oh)
(1) Straight chain peg modifier
mPEG-SC, mPEG-SCM, mPEG-SPA, mPEG-OTs, mPEG-SH, mPEG-ALD, mPEG-butyrALD, mPEG-SS
(2) Bifunctional peg modifier
HCOO-PEG-COOH, NH2-PEG-NH2, OH-PEG-COOH, OH-PEG-NH2, HCl·NH2-PEG-COOH, MAL-PEG-NHS
(3) Branched peg modifier
(mPEG)2-NHS, (mPEG)2-ALD, (mPEG)2-NH2, (mPEG)2-MAL
8. Biotinylation
Biotin can be separated from avidin or streptavidin with the separation strength close to covalent bond. Biotin labeled peptides are commonly used in immunoassay, histocytochemistry and fluorescence based flow cytometry. The labeled anti biotin antibodies can also be used to isolate biotinylated peptides. Biotin markers are often linked to the lysine side chain or N-terminal. In general, 6-aminocaproic acid is used as a bond between peptide and biotin, which can be used to separate substrates sensitively and better when there is steric hindrance.
9. Fluorescent label
Fluorescent markers can be used to track the polypeptides in living cells, and also can be used to study enzymes and mechanism of action. Tryptophan (TRP) is fluorescent, so it can be used as an internal marker. The emission spectrum of tryptophan depends on the external environment and decreases with the decrease of solvent polarity. This property is very useful for the detection of peptide structure and receptor separation. The fluorescence of tryptophan can be quenched by protonated aspartic acid and glutamic acid, which may limit its application. Dansyl chloride group (dansyl) is highly fluorescent when separated from amino groups, and is often used as a fluorescent marker for amino acids or proteins.
Fluorescence resonance energy conversion (FRET) is very useful for the study of enzymes. When FRET is used, the substrate polypeptides often contain a fluorescent marker group and a fluorescence quenching group. The fluorescent group of the mark will be quenched by the quencher through non photon energy transfer. When the polypeptide is dissociated from the enzyme under discussion, the marker group will emit fluorescence.
10. Clathrate peptide
The cage peptide has an optically removable maintenance group, which can block the separation of peptide and receptor. When exposed to UV, the peptide will be activated to restore its affinity to the receptor. Since the optical activation can be controlled by time, amplitude or position, the cage peptide can be used to study the response of intracellular seizures. 2-nitrobenzyl and its derivatives are the most commonly used maintenance groups in cage peptide synthesis. They can be introduced into the amino acid derivatives maintained in peptide synthesis. Amino acid derivatives that have been developed depend on amino acid, cysteine, serine and tyrosine. However, aspartic acid and glutamic acid derivatives are not commonly used because they are easy to cyclize during peptide synthesis and dissociation.
11. Peptide (map)
Short peptides are usually not immune and need to be coupled with carrier proteins to produce antibodies. Multi antigenic peptide (map) is composed of several identical peptides linked to lysine nucleus, which can specifically express high-efficiency immunogen, and can be used to prepare peptide carrier protein coupling bodies. Map peptide can be synthesized step by step on map resin by solid phase synthesis. However, incomplete coupling may result in loss or truncation of peptide chains on some branches, so it does not show the properties of the original map peptide. As an alternative, peptides can be prepared and purified separately and then coupled to map. The peptide sequences linked to the peptide center are clear and easy to characterize by mass spectrometry.
Conclusion:
Peptide modification is an important way to design peptides. Chemically modified peptides can not only maintain high biological activity, but also effectively prevent the defects of immunogenicity and toxicity. At the same time, chemical modification can give some new excellent properties to peptides. In recent years, the application of C-H activated wrist to stop the late modification of peptides has also been developed rapidly, and many important achievements have been made.
If the original article is reprinted, please indicate the source( www.gotopbio.com )”


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
+86 15021993094
Follow Us:




 Pharma Sources Insight July 2025
Pharma Sources Insight July 2025


