May 09, 2024
Tag:
The "Waterloo" of folic acid molecule in targeted Drug Delivery
The active targeted delivery strategy has been proposed as early as the 1980s, aiming at the surface modification specific target molecules of the drug delivery system, by identifying the corresponding target, guide the modified nanomedical drugs to cross the physiological barrier and increase the drug distribution at the focal site. The key to this strategy is to find the right "magic bullet". Among them, folic acid is undoubtedly a star molecule, which is often used as a modification on the surface of various drugs to realize active targeting by recognizing folic acid receptors highly expressed in tumor tissues. There are tens of thousands of publications based on folic acid targeting. Over the past 50 years, several folate-targeting drugs have entered clinical trials. Unfortunately, with the exception of a few folate-probes used to aid surgical navigation, all clinical studies of folate-targeted therapeutics have failed (e.g. Vintafolide, EC150), casting a shadow over the clinical translation of folate-targeted therapeutics. In the field of basic research, folic acid targeting is also controversial, mainly reflected in the inconsistent results in vivo and in vitro. The results of in vitro cell experiments are good, but once in vivo, the targeting effect is greatly reduced. Therefore, clarifying the delivery process of folic acid molecule-mediated targeted delivery system in vivo and revealing the regulatory mechanism related to its delivery are important steps to promote the development and clinical transformation of this type of drug.
In this paper, the team of Professor Zhan Changyou of Fudan University and Associate Professor Wang Huan of Naval Medical University have made a series of work in the field of folate-targeting nanomedicine in recent years.
Origin: Specific adsorption of natural IgM
The delivery process of drug delivery system in the body is extremely complex, and many factors determine its fate in the body. In recent years, the role of plasma proteins in drug delivery systems has received more and more attention. Take liposomes as an example. During blood circulation, liposomes adsorb plasma proteins on their surface to form a protein crown, thus changing the surface properties of liposomes. For example, the fate of liposomes in vivo is negatively regulated by increasing the particle size of liposomes, shielding surface potential, and enhancing the recognition of mononuclear macrophage system through adsorption of opsonins. In particular, active targeting of liposomes can significantly increase the adsorption of plasma protein on the surface of liposomes after modification of target molecules, seriously affecting its targeting, pharmacokinetics, immunogenicity and other properties in vivo.
In the team's previous research, it was found that the natural immunoglobulin M (IgM) in the blood would be widely adsorbed on the surface of various targeted liposomes, resulting in rapid clearance of liposomes. Among them, folic acid liposomes had the highest IgM adsorption, which attracted the attention of the team. There are two main types of natural IgM, the first is free IgM, that is, sIgM, which is usually a pentamer of five IgM monomers connected by disulfide bonds and J chains, with a molecular weight of about 950 kD. It contains 10 Fab segments and 5 Fc segments, and its antigen binding ability and complement activation ability are higher than IgG. sIgM is mainly distributed in the blood and is an important part of the body's innate immune system. The second is the membrane-bound type, mIgM, which is mainly expressed on the surface of B cells in the form of monomer and constitutes the B cell receptor complex, which regulates the humoral immune function of the body.
Based on the specific binding of natural IgM to folic acid liposomes, as well as the biological function of natural IgM, the team explored the black box of folic acid targeted delivery in vivo by studying the interaction of folic acid with sIgM and mIgM.
Sound the alarm: Natural IgM is a key negative regulator of the performance of folic acid targeted delivery systems in vivo
The team first found that sIgM had a specific effect on folate modified liposomes and was positively correlated with the degree of folate modification. This results in a large number of IgM in the blood adsorbed on the surface of folic acid liposomes, causing a series of side effects.
First, sIgM caused the loss of binding activity of folate liposomes. By investigating the targeting of subcutaneous tumor in human gastric cancer cells (SGC-7901, folic acid receptor positive expression), folic acid modification did not increase the targeting of liposomes in tumors, but increased the uptake of liposomes in macrophages at the tumor site.
Second, the presence of natural IgM results in poor blood dynamics of folate liposomes. Moreover, the phenomenon of Accelerated Blood Clearance (ABC) exists. After FA-sLip injection in advance, a large amount of IgM antibodies are generated in the blood, resulting in rapid clearance in the blood after the second injection of folate liposome, and the area under the curve is reduced by nearly 17 times.
Third, the specific binding of natural IgM and folic acid liposomes in human blood is also established, and the binding force is stronger than that of rats and mice. It can also lead to sIgM adsorption, activation of complement and other side effects. In view of the different concentrations of sIgM in patients with chemotherapy, special attention must be paid to the actual clinical administration of folic acid liposomes in the future, and sIgM can be used as an indicator to develop a reasonable administration plan. The results are published in ACS Nano (2020, 14(11), 14779-14789).
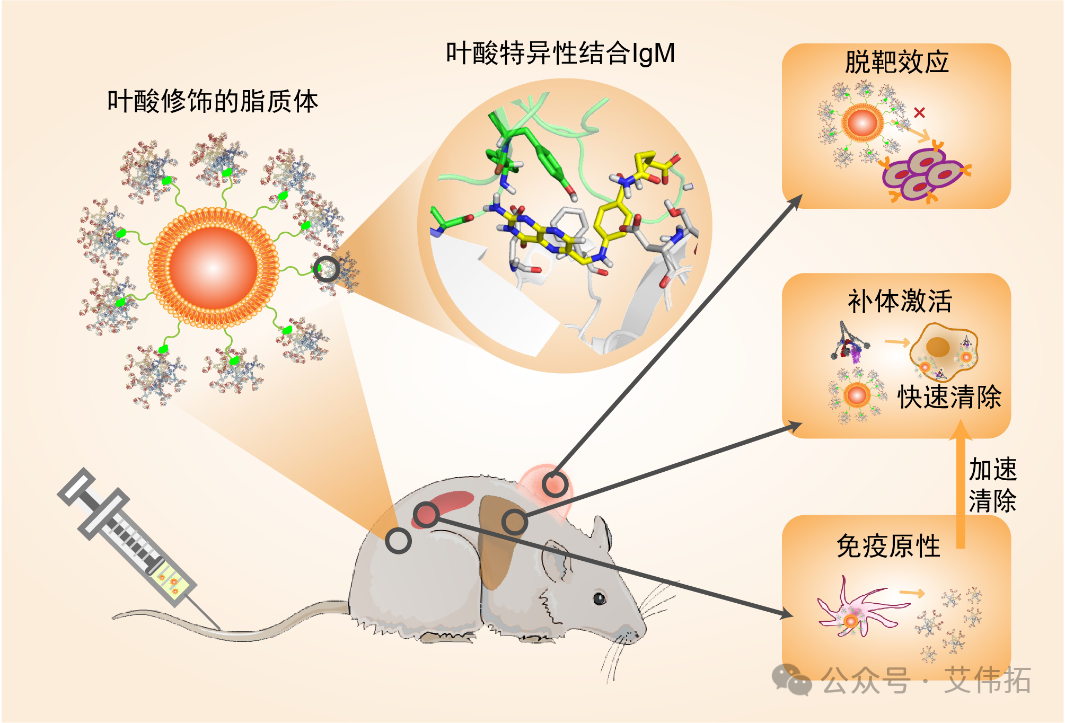
Figure 1. Schematic diagram of negative regulation of sIgM on the in vivo performance of folic acid liposomes [1]
The right medicine: Lipid nanodisks effectively improve the performance of folic acid targeted delivery in vivo
sIgM adsorption-mediated complement activation is the key reason for the poor performance of folate liposomes, so the right medicine should be used. The team cleverly made use of the unique topography of the nanodisk to limit the IgM adsorbed by it to the narrow edge region of the nanodisk, resulting in sIgM unable to show the antigen binding state, hiding its binding site with complement protein C1q, inhibiting its ability to bind complement protein, and effectively avoiding the side effects such as complement activation caused by sIgM adsorption. The in vivo performance of folic acid targeting vector was significantly improved. The research results are published in Nano Lett (202,22(16): 6516-6522).
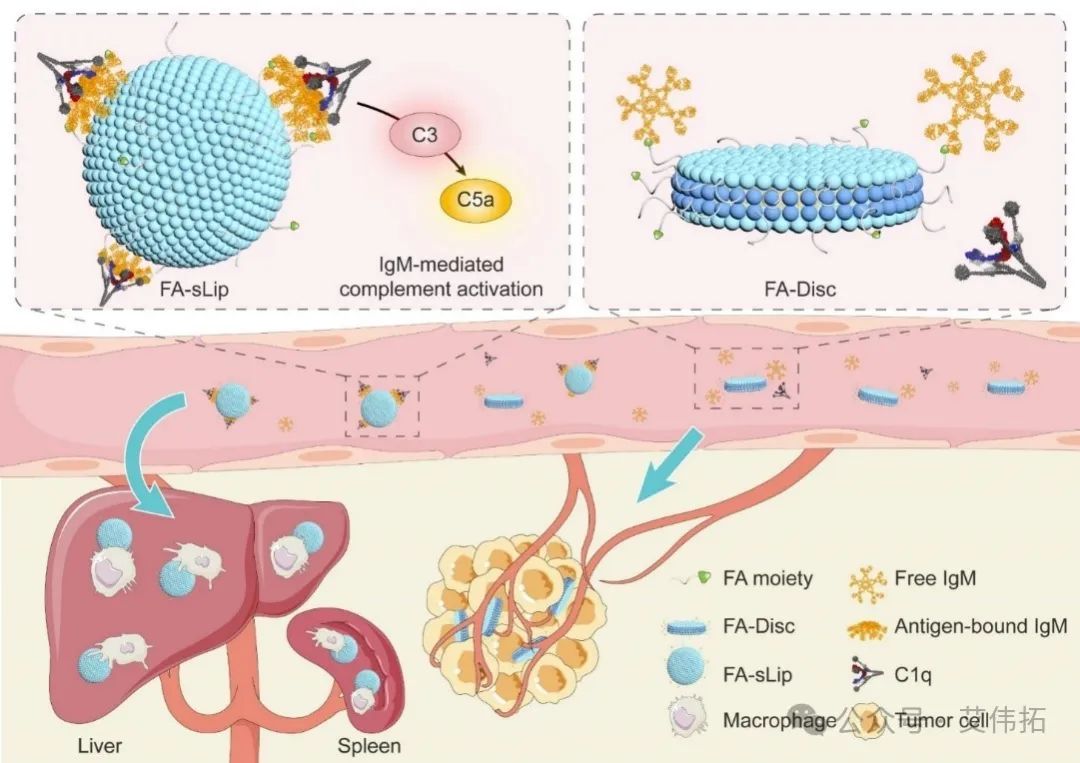
Figure 2. Schematic diagram of folate lipid nanocords effectively improving in vivo performance by circumventing SIGM-mediated complement activation [2]
Trend: Natural IgM enables efficient antigen delivery of nanovaccines
sIgM negatively regulates the targeted delivery of traditional therapeutic drugs. However, appropriate immunogenicity enhancement is beneficial to improve the immune response to vaccine drugs. Given the inevitable adsorption of IgM by folic acid nanoparticles in the body and the multiple functions of IgM in immune response. The team proposed a natural IgM "hitchhiking" strategy to actively and accurately regulate the structure and function of the IgM protein crown on the surface of the nano-vaccine according to the situation, enabling the nano-vaccine to target spleen marginal B cells in vivo, thereby improving the delivery efficiency of the nano-vaccine in vivo. The work is published in J Control Release (2024, 368:208-218).
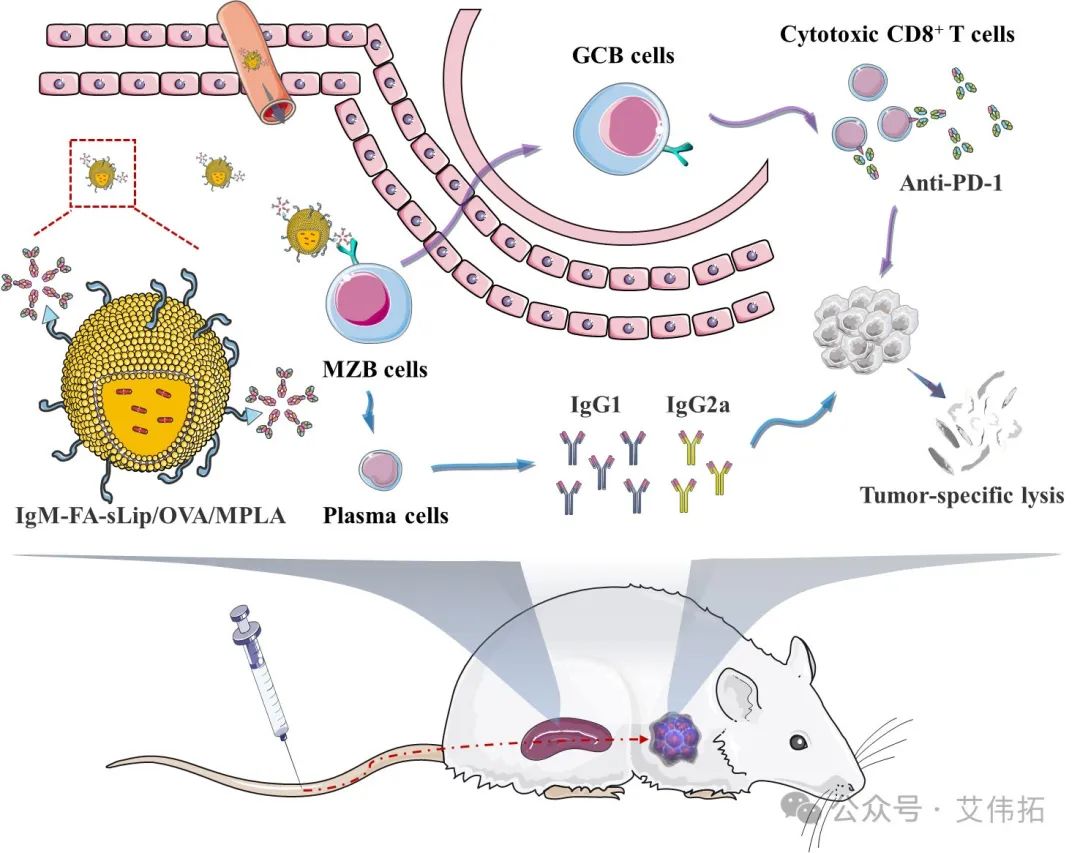
FIG. 3. Design and pharmacodynamic mechanism of tumor nanovaccine based on natural IgM hitchhiking strategy [3]
New approach: Original discovery of folic acid's potential immunosuppressive function
The team found that folic acid specifically binds to the Fc domain of the IgM, thereby enabling FA to target the mIgM-BCR complex of B cells at the splenic margin. Folic acid can induce B cell dysfunction by disrupting the stability of the mIgM-BCR complex and blocking the transmission of antigen signals, thus interfering with the immune response. Oral administration of folic acid, or direct covalent binding of folic acid to biomacromolecule drugs, can induce immune escape and alleviate the production of anti-drug antibodies against biomacromolecules. Using folic acid as a safe and effective immunosuppressant, this study opens up a new way to improve the clinical efficacy of therapeutic biologics. The work is published as a back cover article in Acta Pharm Sin B (2024:14(2):808-820).
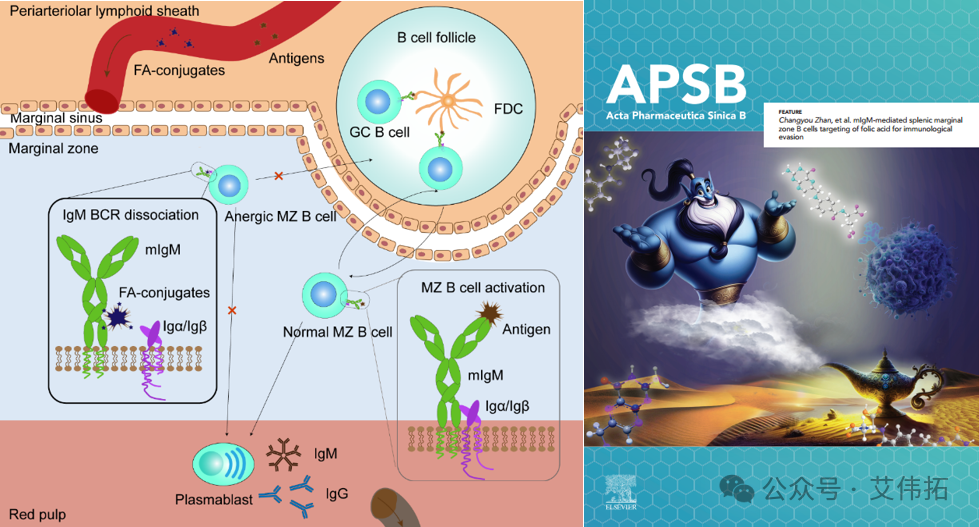
Figure 4. Schematic diagram of B cell incapacitation induced by folic acid [4]
peroration
In recent years, the team has been conducting research on the immune interaction between natural IgM and folic acid and its modified drugs, and related academic ideas have been published in Adv Sci (2023,10(20):e2301777) in the form of a review. The team proposed that natural IgM can be used as a key plasma protein to guide folate targeted drug innovation and precision medicine, and the series of work has filled the theoretical system blank in the study of nanomedicine delivery in vivo to a certain extent.
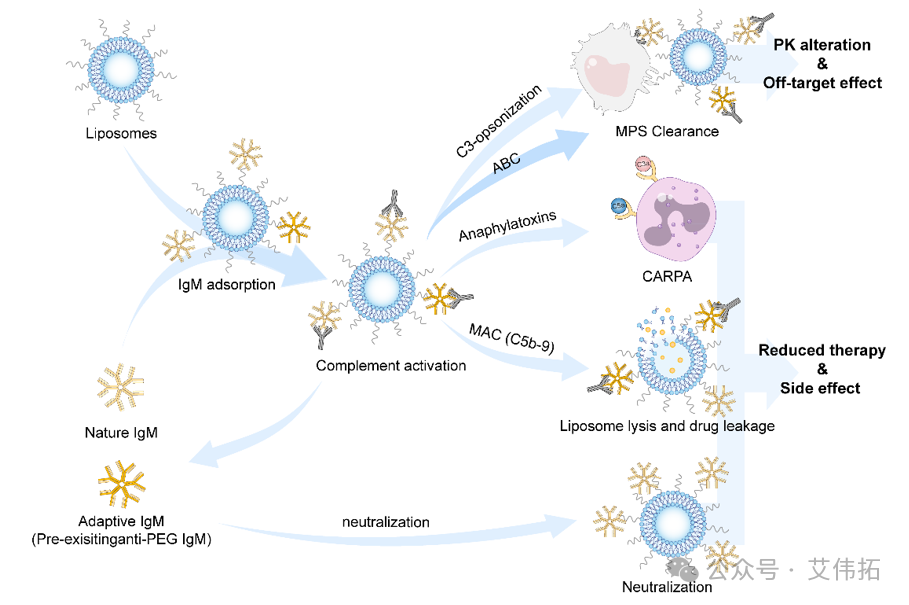
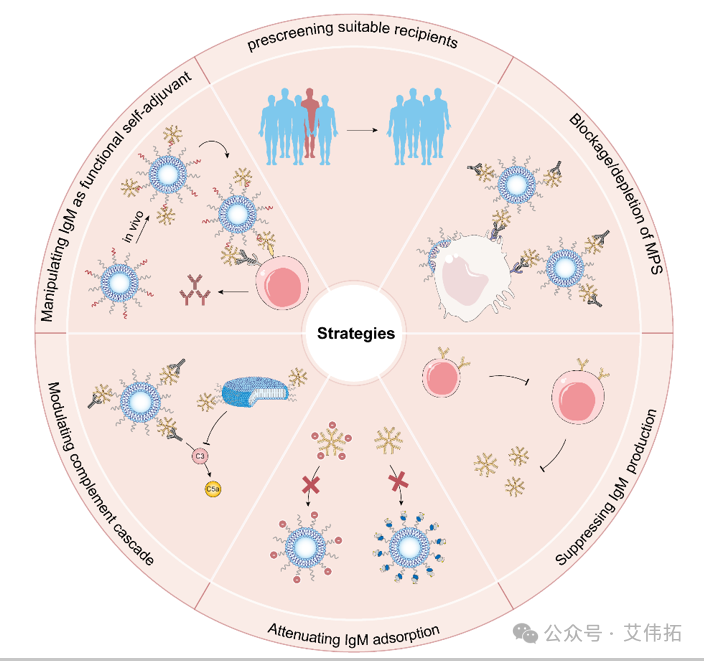
Figure 5. Natural IgM can be used as a key plasma protein to guide folic acid targeted drug innovation and precision medicine [5]
The series of studies used high quality pharmaceutical grade phospholipids provided by AVT. Since its establishment in 2007, Avito has always been committed to serving the Chinese pharmaceutical industry, providing special excipients and high-quality services for high-end pharmaceutical products. A library of 100+ varieties of pharmaceutical excipients has been built, of which 40+ have completed CDE registration, and 16 varieties have been applied in injectable formulations on the market through associated review.
After nearly 20 years of hard work, AVT has realized the supply of all excipients in the field of liposome/nucleic acid delivery, which strongly supports the development and production of domestic complex preparations and domestic and foreign declarations. Welcome to inquire!
AVT, nucleic acid delivery/liposome full excipients supply!

艾伟拓产品团队将持续为您分享艾基金高分文献解读,尽请期待!
参考文献
1. Wang Huan#, Ding Tianhao#, Guan Juan, Liu Xia, Wang Jin, Jin Pengpeng, Hou Shuangxin, Lu Weiyue, Qian Jun, Wang Weiping, Zhan Changyou*. Interrogation of Folic Acid-Functionalized Nanomedicines: The Regulatory Roles of Plasma Proteins Reexamined. ACS Nano 2020, 14(11): 14779-14789.
2. Wang Huan#, Lin Shiqi#, Wang Songli, Jiang Zhuxuan, Ding Tianhao, Wei Xiaoli, Lu Ying, Yang Feng, Zhan Changyou*. Folic Acid Enables Targeting Delivery of Lipodiscs by Circumventing IgM-Mediated Opsonization. Nano Lett 2022,22(16):6516-6522.
3. Wang Huan#, Wu Xiying#, Sun Yuhan#, Liu Anze, He Yingying, Xu Ziyi, Lu Ying*, Zhan Changyou*. A natural IgM hitchhiking strategy for delivery of cancer nanovaccines to splenic marginal zone B cells. J Control Release 2024,28;368:208-218.
4. Wang Huan#, Jiang Zhuxuan#, Guo Zhiwei#, Luo Gan, Ding Tianhao, Zhan Changyou *. mIgM-mediated splenic marginal zone B cells targeting of folic acid for immunological evasion, Acta Pharm Sin B 2024, 14(2):808-820.
5. Wang Huan#, Lin Shiqi#, Wu Xiying#, Jiang Kuan, Lu Huiping, Zhan Changyou*. Interplay between Liposomes and IgM: Principles, Challenges, and Opportunities. Adv Sci 2023,10(20):e2301777.
Thanks to Associate Professor Wang Huan, Naval Medical University


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
+86 15021993094
Follow Us:




 Pharma Sources Insight July 2025
Pharma Sources Insight July 2025


