January 21, 2021
Tag:
When dissecting the structure and function of proteins, the most important thing is how to obtain high purity proteins.The most common way nowadays is the affinity tagging system, which has already become an indispensable special tool in the detection and purification of recombinant proteins.In addition, it is likely to have beneficial effects on the physicochemical properties of the overall target protein, and even increase the production of recombinant proteins.Affinity tags have various classification methods.According to the size of the content, it can be divided into two categories: small molecule short peptide label and protein label.Gutuo Xiao-Editor has specially written a series of articles on "Small Molecule Short Peptide Labels in Detail" for you, hoping to assist you in selecting suitable protein labels for your own research topics.
At present, FLAG-tag is another widely used affinity tag besides His-tag.FLAG-tag is widely used in various expression systems, such as E. coli, yeast and mammalian cells.FLAG-tag consists of eight amino acid residues and the coding sequence is Asp-Tyr-Lys-Asp-Asp-Asp-Asp-Asp-Lys (D-Y-K-D-D-D-K).Because of its small content, only 1.01 kDa, it is not easy to hide other protein epitopes and domains in the fusion protein, and it is not easy to change the role, metabolism or transport of the fusion protein.
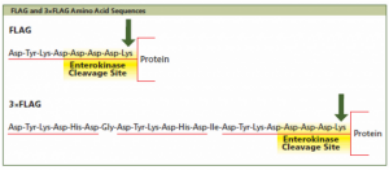
Four linked aspartates in the tag coding sequence give all tags a pure natural hydrophilicity.In this way, it is very easy to locate on the surface of the fusion protein, which facilitates the use of antigens for detection.In particular, the aromatic ring has a very large efficacy in antigen-antigen binding, so the second residue of this label was deliberately designed to be tyrosine.Since FLAG-tag itself is immunogenic, it has three specific monoclonal antibodies, namely M1 monoclonal antibody, M2 monoclonal antibody and M5 monoclonal antibody.This facilitates the detection and purification of fusion proteins.However, FLAG-tag and specific antigen binding were not as strong as those of His-tag and Ni2+-NTA or other affinity tags and detection systems, and the purity of the fusion protein was only about 90% (Terpe, 2003).But it doesn't matter, you can also design three FLAG-tags together (DYKDHDG-DYKDHDI-DYKDDDK), improve the combination of antigen and the sensitivity of detection.Experiments have confirmed that 3FLAG can even detect fusion proteins of a flying magnitude, which is about 20 to 200 times higher than all other systems, especially suitable for the detection of proteins with low and medium expression levels in mammalian cell expression systems.
At the same time, FLAG-tag contains a cleavage site (D-D-D-K-X1) for enterokinase.Experiments have demonstrated that X1, an amino acid residue, affects the cleavage efficiency of intestinal kinase.It happens that when Lysine is in the position of X1, the cleavage efficiency reaches 85%, second only to alanine and methionine (Einhauer & Jungbauer, 2001).Typically, FLAG-tags are placed at the N-terminus, which, when cut off by enterokinase, restores the original sequence and structure of the fusion protein.
Write to the end:
Flag-tag polypeptides are used as organic and pharmaceutical intermediates.Gutuo Biotechnology Co., Ltd. is a manufacturer specializing in polypeptide synthesis and polypeptide customization. It also supplies Flag-tag polypeptide raw materials. Companies that need to purchase APIs are welcome to purchase them.
For any requests of Peptide for research purpose, welcome to contact us. www.gtpeptide.com , sales1@gotopbio.com.


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
Follow Us:




 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025


