December 25, 2020
Tag:
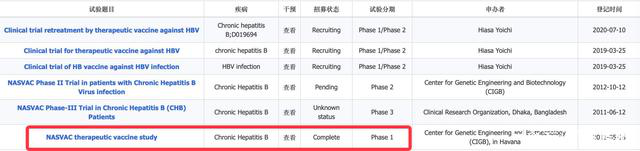
At the just to 2020 American annual meeting for the study of liver diseases (aasld2020), Japanese researchers presented an update on nasvac, a therapeutic waiting list vaccine.The development of this waiting list vaccine, which was first in human phase I clinical studies, started in May 2011, when it could be interrogated for registration information.
Hepatitis B protein / peptide or live vector development, durability is overall good, temporarily weak reactions become difficult.
According to a previous small healthy introduction, nasvac, a therapeutic waiting vaccine containing hepatitis B surface antigen and core antigen, reduced serum HBV DNA levels more than peg IFN therapy in an open label, phase III, randomized, treatment-controlled clinical trial.Around may 2020, studies in another Tupaia model showed that intranasal immunization with hbs-s or hbs-l combined with Hbc formulated with CVP could lead to powerful IgG, IgA, neutralizing antibodies and HBV protein specific IFN - γ immunization countermeasures.
In addition to the vaccine candidate nasvac, dv-601 is a therapeutic vaccine composed of HBS and HBC antigen that, in its phase I study, was found to be safe and durable, with antiviral responses occurring.Heptcell is an immunotherapeutic synthetic research drug consisting of 9 peptides (derived from highly conserved regions of three different HBV antigens (polymerase, core and surface)) designed to stimulate CD4 + CD8 + T cells from HBV carriers, irrespective of HLA background.
In its phase I clinical study, heptcell immunotherapy using ic31 (TLR9 stimulant) adjuvant was found to be of good durability, with increased T cell responses to HBV, but no apparent effect on hepatitis B surface antigen.Some of the above presentations, coupled with the previous scientific popularity of gs-4774 are protein / peptide vaccines.There is also a live vectored vaccine, tg1050, in the world that can be individually analyzed for mechanism of action and trial progress.
Tg1050 is an adenovirus based therapeutic vaccine that demonstrates immunogenicity and antiviral effects in mice (a preclinical animal model) against three HBV proteins (polymerase, core and surface antigens).In a phase I clinical trial for chronic hepatitis B receiving nucleoside (NA) therapy, tg1050 showed a good safety profile, guided HBV specific cellular immunity, supporting further clinical evaluation, especially combination therapy.
The live vectored vaccine another is aic649, whose phase I clinical trial found that this inactivated paravaricella virus (ippvo) formulation had good durability and resulted in higher IL-1 β, IL-6, IL-8, and IFN - γ grades, as well as lower IL-10 plasma grades.Into the early 1990's, the world first reported that plasmid DNA induced plasmid encoded antigen immune responses, so DNA vaccines became gradually a new immunization method and a rapidly developing field of vaccine technology.
Plasmid DNA has a shorter half-life than injected protein antigens, can provide tissue manifestation of the antigen over a longer period of time, and therefore may better prime the immune system.Pcmv-s2. S is a DNA vaccine encoding HBV small (s) and medium (pre2 + s) envelope proteins, which was presented by Moroccan and Japanese investigators on 11 may 2020 in vaccines.In a phase 1 clinical trial in 10 chronic HBV carriers, it was durable and could activate or restore T-cell responses in chronic HBV carriers, but this effect was short-lived and weak.
The efficacy of pcmv-s2. S-DNA in preventing the recurrence of hepatitis B virus was then investigated in a phase 1 / 2 clinical trial of 70 patients treated effectively with nucleoside drugs.The findings showed that pcmv-s2. S was safe but could not control the recurrence and recovery of HBV immune response.Therefore, to date, modern science has achieved considerable progress, but the direction of protein / peptide vaccines and the direction of live vector vaccines, DNA vaccines are very resistant, which is also the view of scientific research endeavors, back to the opening of the development of the vaccine candidate nasvac (protein / peptide vaccines) over a long time span, mainly based on the above reasons.
For any requests of Peptide for research purpose, welcome to contact us. www.gtpeptide.com , sales1@gotopbio.com.


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
Follow Us:




 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025


