November 25, 2024
Tag:
Thanks to the high selectivity and volume removal rate of Protein A affinity chromatography resins for antibodies, as well as their robust impurity removal effect, Protein A affinity chromatography resins serve as the industry standard for antibody drug manufactruing.
Due to the limitations of Protein A's binding capacity and the high costs associated with Protein A resins, increasing the lifetime of resins becomes particularly important. It is generally believed that the repeated use lifetime of Protein A resins is affected by two factors: resin hydrolysis and resin fouling.
Protein A resin hydrolysis refers to the degradation of the ligand when exposed to cleaning solutions (such as sodium hydroxide) for an extended period of time. Protein A resin fouling occurs due to the continuous accumulation of impurities on the surface of the resin caused by the use of ineffective cleaning solutions.
Resin Ligand Hydrolysis
To detect the hydrolysis rate of Protein A ligand, unused resins were subjected to a continuous cycle of alkaline solutions of different concentrations for over 250 hours. The concentrations and temperatures of sodium hydroxide were evaluated, and the decrease in DBC (Dynamic Binding Capacity) of the resins was used as an indicator of the hydrolysis rate of Protein A resins. As shown in Figure 1, higher concentrations of sodium hydroxide resulted in a faster ligand hydrolysis rate. Additionally, lower temperatures mitigated the extent of ligand hydrolysis to a certain degree.
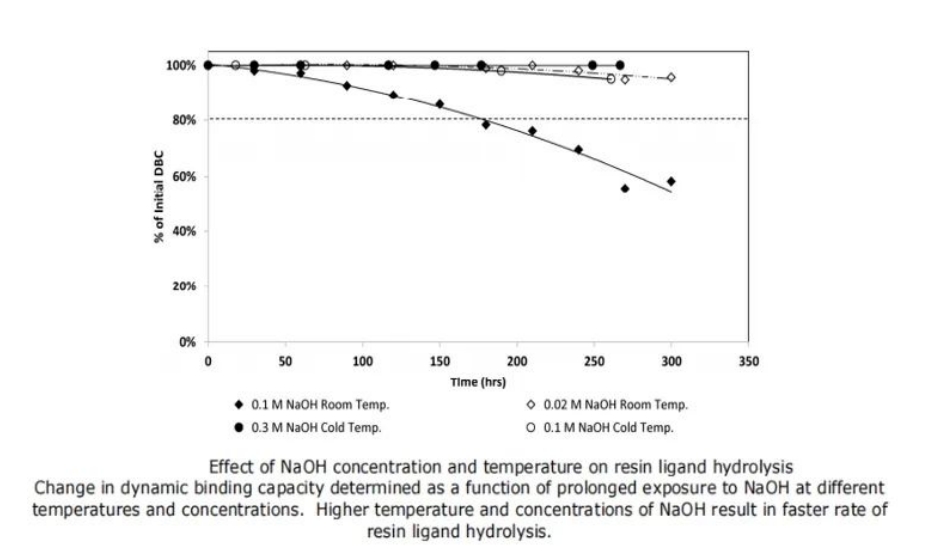
Figure 1: Effect of Sodium Hydroxide Concentration and Temperature on DBC
The data indicate that ligand hydrolysis rates can be mitigated under low-temperature conditions, and the DBC of different molecules exhibits varying degrees of sensitivity to low temperatures. Seven model proteins were used as representatives to investigate the DBC of different molecules at different temperatures, where Molecule B is an Fc fusion protein with a pI of 7.9, and the remaining are IgG1 monoclonal antibodies with pI ranging from 6.5 to 8.9. As shown in Figure 2, it can be observed that different molecules exhibit varying degrees of capacity reduction at low temperatures, which to some extent offsets the benefits of low temperatures in mitigating ligand hydrolysis. Additionally, performing Protein A chromatography cleanup steps in a cold environment during production may lead to outgassing effects on the chromatography column, which is not conducive to manufacturing. Therefore, using sodium hydroxide for cleaning at low temperatures is not an ideal solution.
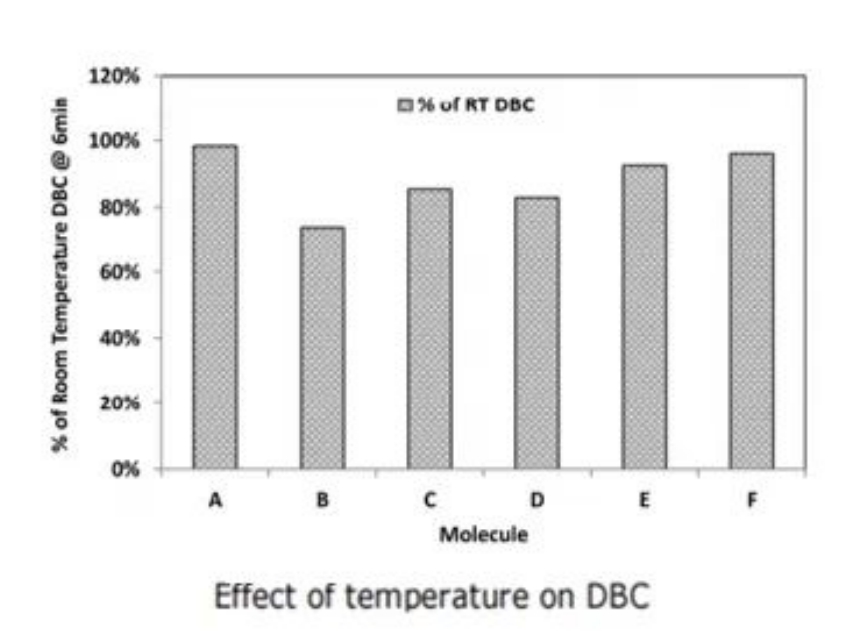
Figure 2: Use of Additives to Delay Ligand Hydrolysis
Different additives have varying effects on delaying ligand hydrolysis. Taking Molecule E as a representative, sucrose, ethylene glycol, and propylene glycol were added as additives to sodium hydroxide, and their DBC after cleaning solution cycling was evaluated. As shown in Figure 3, the additives have a certain effect on delaying ligand hydrolysis. Specifically, the ligand stability of propylene glycol/sucrose and ethylene glycol was improved by 2 times and 3 times, respectively, compared to the baseline condition of 0.1 M NaOH.
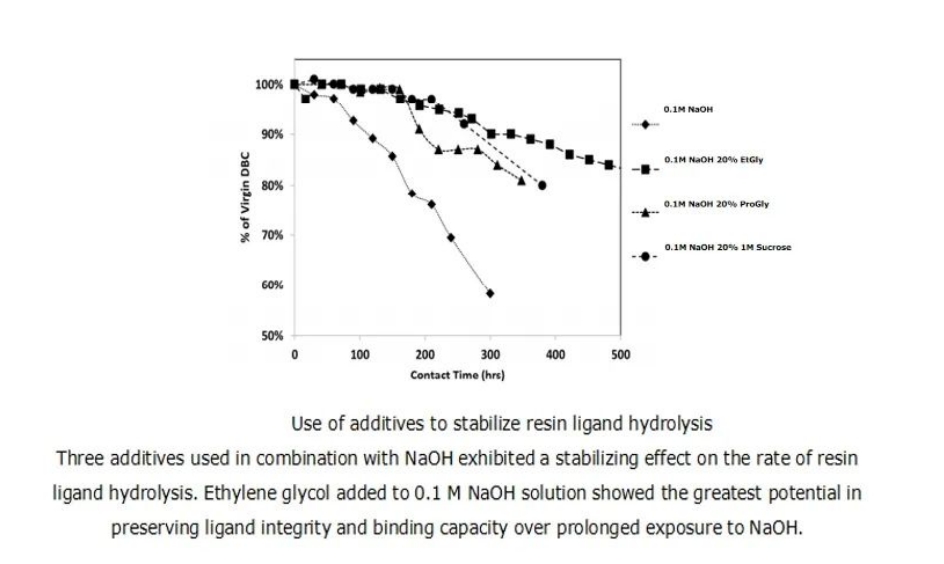
Figure 3: Comparison of Resin Fouling between Alkaline and Non-Alkaline Cleaning Solutions
Three molecules (one monoclonal antibody and two Fc fusion proteins) were selected as representatives to study resin fouling after cleaning. To maximize the fouling of the chromatography column, clarified harvest solutions of the three representative molecules were subjected to 10 cycles on a Protein A chromatography column, with each cycle only reaching the elution step without performing column cleaning. Afterwards, the chromatography column was disassembled and the resins were separately incubated in different cleaning solutions at different temperatures for 2 hours. The resins were then washed three times with equilibration buffer, and the original resins were used as a control. At this point, the remaining residue on the resins represented uncleaned fouling. The final resin suspension was diluted to 1x SDS PAGE loading buffer, incubated at 80°C for 10 minutes, and the supernatant was collected for SDS PAGE analysis. The more proteins present on the SDS PAGE gel indicated more uncleaned fouling.
As clearly shown in Figure 4, the use of higher concentrations of sodium hydroxide (0.1M and 0.3M, lanes 2, 4, 5) as cleaning solutions significantly outperforms 0.02M (lane 3) in terms of cleaning effectiveness. This trend is consistent across all three test molecules. While 0.3M NaOH performs better for Molecule A compared to 0.1M NaOH, the results are comparable for the other two molecules. Additionally, the non-alkaline cleaning solution at pH 11 (lane 6) shows good cleaning performance for Molecule A but is less effective for the other molecules. This supports the theory that non-alkaline cleaning solutions are not suitable as a platform cleaning strategy. Interestingly, temperature does not appear to affect the efficiency of resin cleaning. Similar results are obtained at both ambient and lower temperatures (lanes 4 and 5) when using a higher concentration of sodium hydroxide (0.3M).
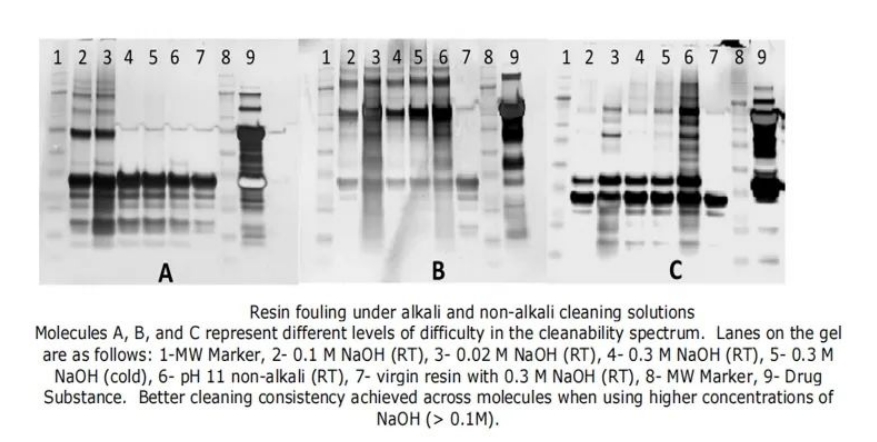
Figure 4
To better characterize the effect of cleaning duration on cleaning efficacy, further experiments were conducted using Molecule B (Fc fusion protein, pI 7.9) as a representative. The resins contaminated with Molecule B were subjected to varying incubation times with cleaning solutions. As shown in Figure 5, with increasing incubation time, fewer proteins are observed on the gel lanes, indicating better cleaning performance. However, increasing the incubation time may lead to more ligand degradation, so both incubation time and ligand degradation should be considered comprehensively to achieve optimal resin lifetime.
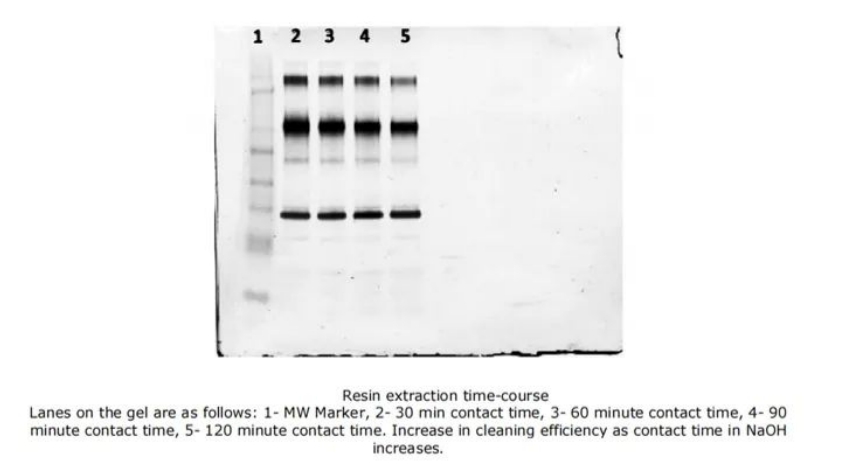
Figure 5: Resin Lifetime Study
Based on the above data, 0.1 M NaOH and 0.1 M NaOH + 20% Ethylene Glycol (EtGly) were selected as cleaning solutions to evaluate their reusability. As shown in Figure 6, the column lifetime nearly doubled when 20% EthGly was added, without any decrease in product quality. The use of ethylene glycol as an additive for cleaning affinity chromatography columns results in a gradual increase in elution volume as the number of cycles increases, but this increase does not affect the corresponding product quality. Therefore, ethylene glycol can still be used as a cleaning additive for affinity chromatography.
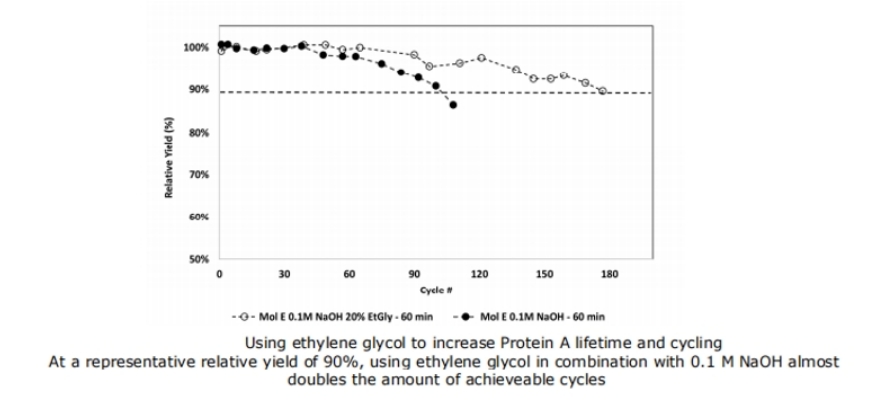
Figure 6
BioLink's MaXtar® ARPA Protein A Affinity Chromatography Resin, utilizes a highly alkali-resistant Protein A ligand with full independent intellectual property rights. It adopts innovative green chemistry bead formation processes, coupling methods, and ligands, resulting in exceptional alkali resistance. When subjected to CIP treatment with 0.5M NaOH for 15 minutes in each cycle, the dynamic binding capacity (DBC) of MaXtar® ARPA retains over 90% of its original capacity after 200 cycles.
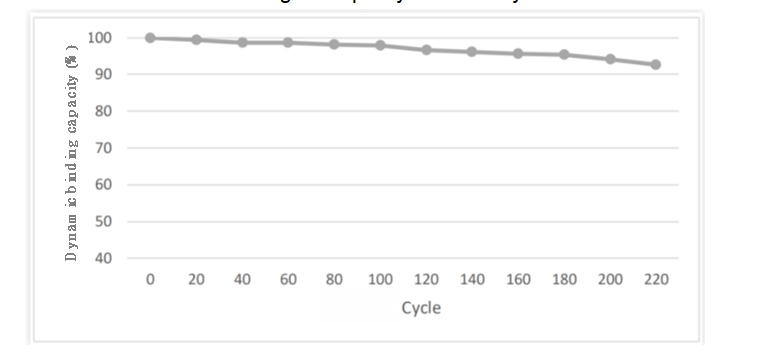
Figure 7
Reference:
Zhang J, Siva S, Caple R, Ghose S, Gronke R. Maximizing the functional lifetime of Protein A resins. Biotechnol Prog. 2017 May;33(3):708-715. doi: 10.1002/btpr.2448. Epub 2017 Mar 20. PMID: 28218470.


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
Follow Us:




 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025


