November 26, 2024
Tag:
ChromaX® Chromatography Resin Family from BioLink
BioLink MaXtar® ARPA Affinity Chromatography Resin and polishing resins have completed domestic replacements in many projects in China, and have successfully entered pilot production phase due to their excellent comprehensive performance and high cost-effectiveness.
At the same time, we have assisted customers in completing domestic replacements and production in other projects, with the largest scale expanded to a 1.6m column production.
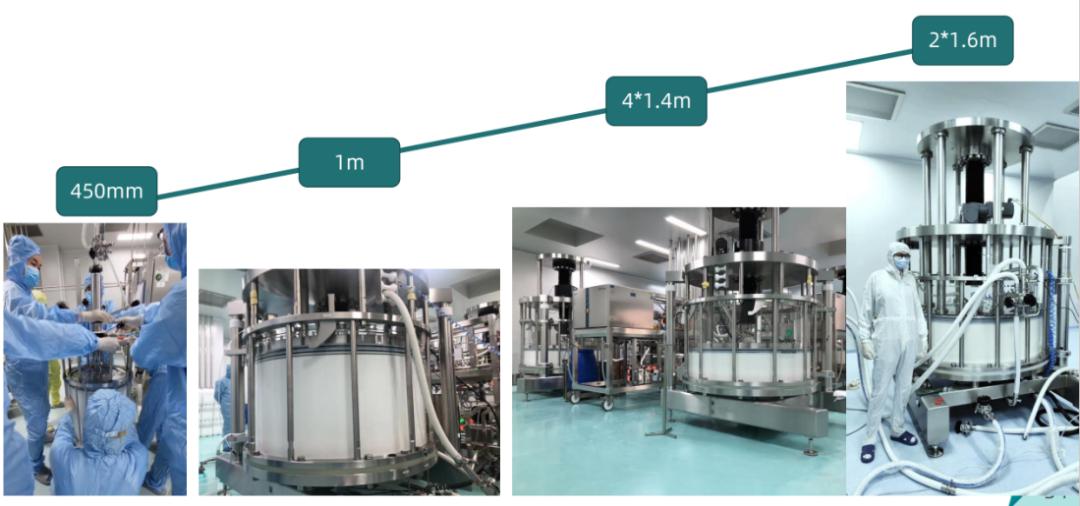
BioLink chromatography resins applied for clinical changes in both China and the United States.
In accordance with the relevant policies and regulations of the National Medical Products Administration, the change of chromatography resin for therapeutic biologics, the change process is summarized as follows:
Establish internal change management system for the enterprise
Assess and manage all changes affecting product quality, and communicate with the quality management department responsible for change control. Based on the stage of the company's products and in accordance with the current regulations, carry out change process control.
Risk Assessment and Implementation of Changes
Changes must undergo evaluation, documentation, and verification to allow the company to demonstrate sufficient knowledge in preparing and managing the impacts of changes. It should be documented and provided to the relevant authorities for their approval before implementation. This includes a detailed description of the change, the reasons for the change, the involved products, the affected production sites or areas, a description of the methods and studies used to assess the impact on product quality changes, and data from these studies (comparative study protocols). It also involves relevant validation plans and data, along with a reference checklist of related Standard Operating Procedures.
Risk Assessment of Chromatography Resin Changes
Given the foundation of scientific knowledge and a thorough consideration of the existing understanding of products and processes, especially Critical Quality Attributes (CQA), Critical Process Parameters (CPP), Key Process Parameters (KPP), and Critical Material Attributes (CMA), the risk assessment of filling material changes is essential. Generally, it is believed that filling material changes impact Critical Material Attributes (CMA), altering the fundamental chemical composition, structure, crosslinking, particle size, pore size, ligands, and other characteristics of the filling material. This, in turn, affects column packing, chromatography processes, and ultimately influences purity and impurities. Therefore, a comprehensive assessment of these impacts is necessary when making changes to chromatography resin.
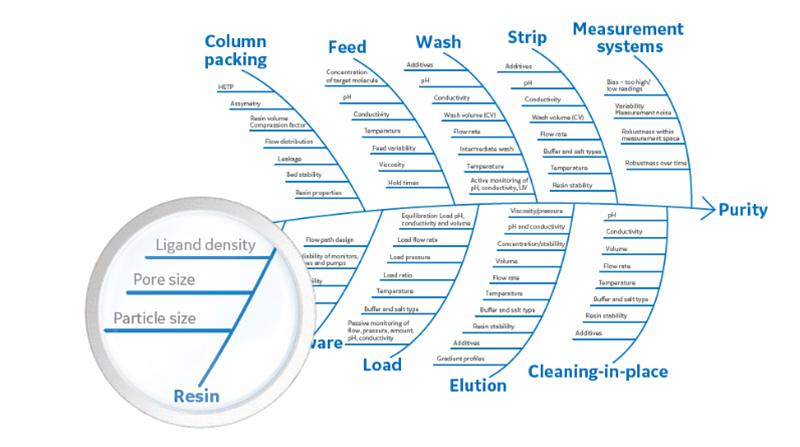
Comparability Studies
The execution of comparability studies is based on the progressive principle of risk. It consists of three levels, with the lowest being pharmaceutical comparability. This involves analyzing the quality attributes of drugs through various dimensions using analytical methods to assess their comparability. If at this level (pharmaceutical comparability), the comparability of process changes cannot be proven, it needs to progress to the next level (non-clinical studies). Non-clinical studies involve conducting animal experiments to compare the pharmacokinetics/pharmacodynamics (PK/PD) and toxicity of drugs before and after process changes. If, at this level, the company still cannot prove comparability, it needs to further progress to the highest level, conducting clinical trials to demonstrate comparability.
Chromatography Resin Change Process Study
Chromatography resin changes generally start with process studies (Scale down), determining possible classifications for changes and conducting production-scale change studies (1-3 batches). Stability studies of the formulation are also conducted. Chromatography resin, being a special critical material, undergoes continuous changes in its key core performance parameters during repeated use in the production process. To ensure that its performance meets process requirements at the end of its lifespan, a material lifespan study must be conducted. If this step involves virus clearance, a virus removal study is also required, typically conducted using a Scale down model.
Submission and Approval of Changes
After completing the studies, it is recommended to communicate with the local drug regulatory department, determine the change declaration process, and submit relevant documents to the regulatory authorities based on the "Drug Registration Management Measures." Chromatography resin changes usually fall under the category of moderate or significant changes and require approval before implementation.


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
Follow Us:




 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025


