September 28, 2020
Tag:
The novel coronavirus pneumonia has spread rapidly worldwide over the past two months. According to the latest real-time statistics from the World Health Organization, up to now, the current global confirmed cases of new crown pneumonia have exceeded twenty million cases, with more than 74 deaths. Italy, France, Australia and other countries have successively released the information of their enfeoffment. Some countries have successively announced the closure of all border crossings and the suspension of all air and sea transportation. Indonesia, the United Arab Emirates and other countries have comprehensively suspended visa free and landing visa services for foreign citizens of all other countries. To some extent, the rapid spread of the epidemic has given human society Communication and global economic development have pressed the "pause" button. However, risks and opportunities will always coexist. In every turmoil, some people will fall down, but there will also be people who will stand up and look for opportunities in the crisis. This is our best counterattack against the epidemic.
On May 1, U.S. local time, FDA authorized the emergency use of the antiviral drug, remdesivir, for the treatment of severe covid-19 adults and children. Although there are limited data on the efficacy and safety of the drug, a study has shown that radcivir can significantly shorten the recovery time of patients, the FDA said. Japan's Ministry of labor, welfare and welfare said it was ready to put the drug into the rapid approval channel, and it is expected to let domestic patients use the drug one week after Gilead submitted its application.
According to the EUA authorization of FDA, redcivir can be used for critically ill patients. In the United States, severe cases are defined as oxygen saturation (SPO 2) ≤ 94% under indoor air conditions or oxygen support, mechanical ventilation and extracorporeal pulmonary membrane oxygenation (ECMO). Gilead science is expanding the supply of the antiviral drug radcivir, which has just been approved by the FDA for emergency use (EUA), this week for new crown patients in U.S. hospitals. Radcivir has to be given intravenously.
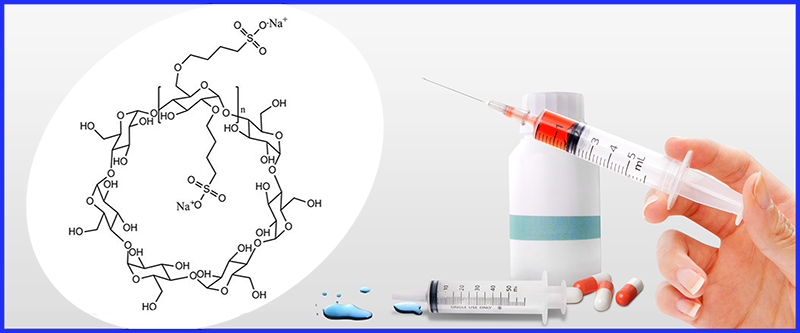
Betadex Sulfobutyl ether sodium produced by Shandong Binzhou Zhiyuan Biotechnology Co., Ltd. is a key excipient for the production of radcivir. As an excipient supplier recognized by pharmaceutical manufacturers at home and abroad, our company is striving to overcome various difficulties and actively cooperate with the R & D and production work of relevant units.


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
Follow Us:




 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025


