January 31, 2021
Tag:
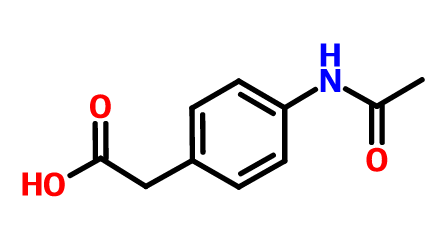
Actarit (abbreviated as ACTA) is an anti rheumatic drug developed by Mitsubishi Chemical Industry Company of Japan. It can inhibit the delayed allergic reaction and improve the early symptoms of rheumatoid arthritis (RA) by reducing the concentration of nitric oxide in serum. It is mainly used for the treatment of RA. However, due to the poor water solubility of Acta, its clinical application is limited.
Hydroxypropyl beta cyclodextrin (hp-b-cd) is a low toxicity, safe and effective drug solubilizer, which has the advantages of high water solubility and improving the release rate of the drug in vivo. The inclusion complex of Acta HP - β - CD was prepared to increase its solubility in water, and then appropriate excipients were added to fill the capsules to achieve good dissolution effect, which provided theoretical basis for further development of new dosage forms of Acta and improvement of its pharmaceutical properties.
Preparation of solid inclusion complex: weigh hydroxypropyl betacyclodextrin and Acta in a beaker according to the molar ratio of 1:1, add anhydrous ethanol to dissolve Acta, add them dropwise into hydroxypropyl betacyclodextrin ethanol solution under stirring condition, and continue stirring for 4 h at room temperature. Take it out, take 60 water bath, remove ethanol by rotary evaporation, grind it fine after vacuum drying, and pass through 80 mesh sieve.
The content of Acta in the inclusion complex was determined. The appropriate amount of Acta was weighed and diluted to the appropriate concentration with anhydrous ethanol solution. The ethanol solution was used as the blank and scanned at the wavelength of 200-400 nm. The results showed that Acta had the maximum UV absorption at 245nm, so 245nm was selected as the detection wavelength. In the test, the powder is easy to leak out, and it is impossible to ensure that the sample is put into the medium at the same time, so the paddle method is selected. When granulating, it is found that the viscosity of water as wetting agent is high, which brings some difficulties to granulation. 95% ethanol is used as wetting agent to solve this problem. According to the Chinese Pharmacopoeia, the dissolution rate should reach 75% at 45min. The results show that the capsules with different prescriptions meet the requirements. Some prescriptions have slight attenuation, which should be related to the continuous supplement of media. The effect of fillers on the dissolution of inclusion compound capsules is small, which may be because the preparation of inclusion compound itself improves the solubility of Acta and accelerates the dissolution rate of drugs, so that the inclusion compound capsules have good dissolution effect no matter what kind of fillers are used. If we want to prepare ordinary capsules, we can use economical starch as excipient to meet the dissolution requirements; if we want to make quick release capsules, we can consider using lactose as filler.


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
Follow Us:




 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025


