A study conducted under the Lung Cancer Master Protocol (Lung-MAP) found that when treated with a combination of ramuzumab (Cyramza) and Keytruda, Patients with advanced NSCLC whose cancer progressed during prior immunotherapy lived significantly longer than when treated with one of the current standard therapies for this cancer. The hazard ratio (80% confidence interval) for overall survival (OS) time for patients in the study group versus those in the standard-of-care group was 0.69 (0.51-0.92). The median OS time in the two groups was 14.5 months and 11.6 months, respectively. The results was published in the Journal of Clinical Oncology.
The study, also known as S1800A, was conducted as part of Lung-MAP, the first lung cancer precision medicine trial supported by the National Cancer Institute (NCI), a division of the National Institutes of Health (NIH), and conducted at NCI’s National Clinical Trials Network (NCTN) and the NCI Community Oncology Research Program (NCORP).
The principal investigator for the S1800A is Karen Reckamp, MD, MS, Director of the Division of Medical Oncology and Associate Director of Clinical Research at Cedars-Sinai Medical Center in Los Angeles. Dr. Reckamp will present the trial results at the ASCO meeting.
"This is the first trial to show a survival benefit of ICI and VEGFR inhibition in patients with advanced lung cancer who have experienced tumor progression in a previous ICI," Reckamp said.
Pembrolizumab is a class of immunotherapy drugs called immune checkpoint inhibitors (ICIs), while ramucirumab is a vascular endothelial growth factor receptor 2 (VEGFR-2) inhibitor, a class of drugs that block the enzyme drug.
The randomized phase 2 S1800A study enrolled 136 eligible patients with stage IV or recurrent non-small cell lung cancer. These patients had previously received ICI therapy. In all cases, the patients' tumors eventually became resistant to these drugs and grew or spread before the patients were enrolled in the S1800A trial.
The trial rapidly accumulated and had a diverse population, in large part because of the strong rollout of the Lung-MAP primary screening protocol. Roy S. Herbst, MD, associate director of the Yale Cancer Center, founder and principal investigator of Lung-MAP, and senior author of the paper, commented, "The unique nature of the Lung-MAP public-private partnership, the breadth and breadth of NCI's NCTN and NCORP, Backed by the diverse nature, we are able to bring these new treatments to patients with advanced lung cancer nationwide at no cost—an important improvement in patient access."
Overall survival was the primary endpoint of the trial. Secondary endpoints included progression-free survival (PFS) and objective response rate (ORR). The researchers found no significant difference in PFS between the two groups (hazard ratio [80% confidence interval]: 0.86 [0.66-1.14]; median: 4.5 months in the ramucirumab plus pembrolizumab group, compared with standard care group) or ORR (22% vs. 28%).
Treatment-related adverse events (side effects) of grade 3 or higher occurred in 42% of patients in the ramucirumab plus pembrolizumab group and 60% of those in the standard-of-care group.
Treatment in the trial control group was chosen by physicians and patients from a group of four standard chemotherapy regimens: docetaxel plus ramucirumab, docetaxel alone, gemcitabine or pemetrexed. About two-thirds of patients receiving standard care received docetaxel and ramucirumab as the most aggressive treatments approved in this setting.
The researchers note that the relatively small sample size of the S1800A means that the results of the trial cannot be considered conclusive, and the combination should be investigated in a larger trial.
"These results represent a potential paradigm-changing scenario with limited options," Reckamp said. "A phase 3 trial of this combination is warranted to better assess its impact."
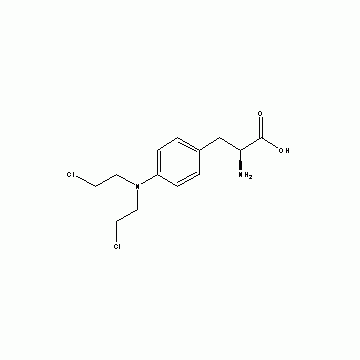
Huateng Pharma, as a leading pharmaceutical intermediates supplier in China, can provide CAS NO.137281-39-1, CAS NO.165049-28-5, CAS NO.1118-89-4, CAS NO.155405-80-4 and CAS NO.3140-73-6 from lab to commercial scale, which can be used as Pemetrexed disodium intermediates. Also, we can provide CDMO services to meet your requirements, combining a high level of competencies, equipment and quality.





 ALL
ALL Pharma in China
Pharma in China Pharma Experts
Pharma Experts Market News
Market News Products Guide
Products Guide Brand Story
Brand Story


















![(5R)-3-[3-Fluoro-4-(4-morpholin
yl)phenyl]-5-hydroxymethyl-2-
oxazolidinone](https://eimg.pharmasources.com/image/product/20240111/7871140085365a7029d2ca200cd83f9bjpg)












 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025








