June 15, 2020
Tag:
SUS (Single Use System) has been widely used in biopharmaceutical field, covering the whole process of liquid mixing, liquid storage, liquid transferring, cell culture, purification, preparation, filling and packaging. SUS has the advantages of operating flexibly, using safely, no risk of cross-contamination, avoiding CIP / SIP, and reducing the verification of cleaning. For new antibody R & D enterprises and CDMO enterprises seeking more efficiency and flexibility, these advantages have great attraction.
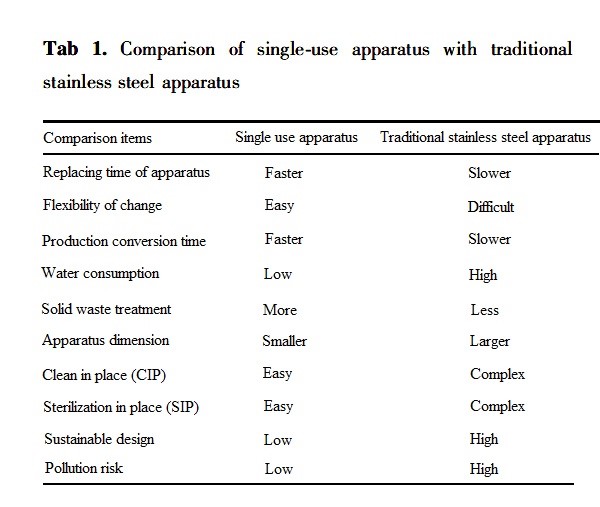
The development and application of single use technology in the upstream stage of biopharmaceutical field is mature enough, with a high popularization rate. However, the use of single use technology in downstream process is relatively less. With the mature application of the upstream process, the development of the downstream process has also made great progress. But whether upstream or downstream process, mainstream equipment and consumables suppliers are all foreign enterprises, and few domestic suppliers are involved. In order to fill the domestic gap about single use technology, Jiangsu Hanbon Sci. & Tech. Co.,Ltd has invested in the research and development of SUS. At present, the Single Use TFF UF / DF system is officially launched.
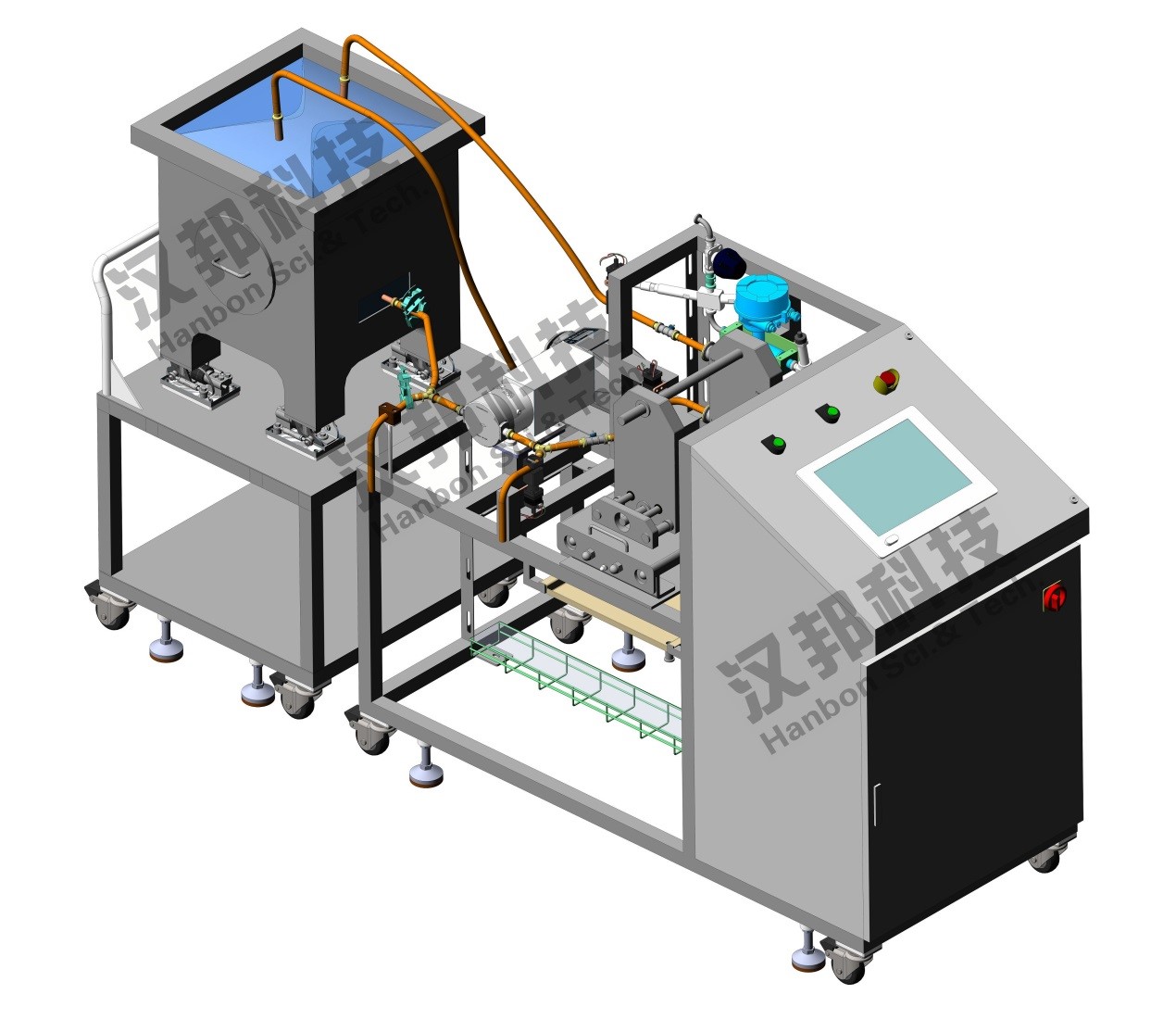
Compared with traditional TFF equipment, the installation, connection and pretreatment of Single Use TFF UF / DF system are simple and convenient. At the same time, the system can save time cost and reduce labor input economically. The system pump head, valves, piping, pressure sensors, flow meters, pH, conductivity, UV detector and other parts in contact with the liquid are disposable replaceable parts. All parts are pre packed sterile in advance and can be directly unpacked and installed before use. CIP verification is not required. So the single use TFF UF / DF system is especially suitable for the applications of multi CDMO or multi product collinear.
Technical Advantages:
1. All parts contacting with liquid can be replaced directly, fast and convenient;
2. Non-stainless steel materials in contact with solution are available with FDA and USP VI certificates;
3. Complete audit trail function. Files, data and operation logs can be traced;
4. Compact design and minimum recyclable volume can achieve higher concentration multiple;
5. Use the same software as non-SUS, so the operator can learn fast and use easily.
For more information about Single Use TFF, please contact the Macromolecular Business Department of Hanbon Sci & Tech Co.,Ltd, Email: gljia@hanbon.com.cn. We are also researching and developing the single use chromatography system, and there will be significant progress in the near future. I will report to you as soon as possible.


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
Follow Us:




 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025


