September 27, 2020
Tag:
Many animals and plants (such as snakes, scorpions, sea anemones, etc.) have acquired the ability to produce venom in the process of natural selection and evolution to prey or protect themselves. Peptide toxins in toxin proteins can specifically act on ion channels, cell membrane surface receptors and other life related proteins. Because of the high specificity and affinity of the interaction between toxins and target proteins, toxins can be used as molecular tools to study the structure and physiological functions of proteins, and to develop drugs for the treatment of diseases.
As early as 2012, French researchers isolated two peptides, mambalgins, from the venom of the black mamba snake, which can relieve pain in mice. Through in-depth study, mambalgin can effectively inhibit the acid sensing ion channel (ASIC) of central nervous system and primary pain receptor, block the signal related to pain in neural pathway, so as to achieve the analgesic effect. Its analgesic effect is comparable to that of morphine, and its side effects are small, so it has the value of drug development.
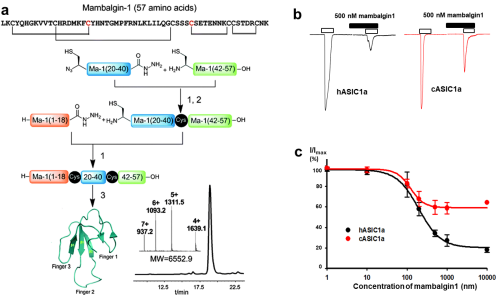
Subsequently, mambalgin 1 toxin with biological activity was successfully prepared by protein chemical synthesis technology in 2014, and the solution structure of mambalgin toxin was analyzed by liquid NMR method. However, how mambalgin toxin can specifically recognize and inhibit the activity of human derived ASIC channels (the inhibitory effects of mambalgin toxin on human and chicken derived ASIC channels are significantly different), and the mechanism remains to be further studied.
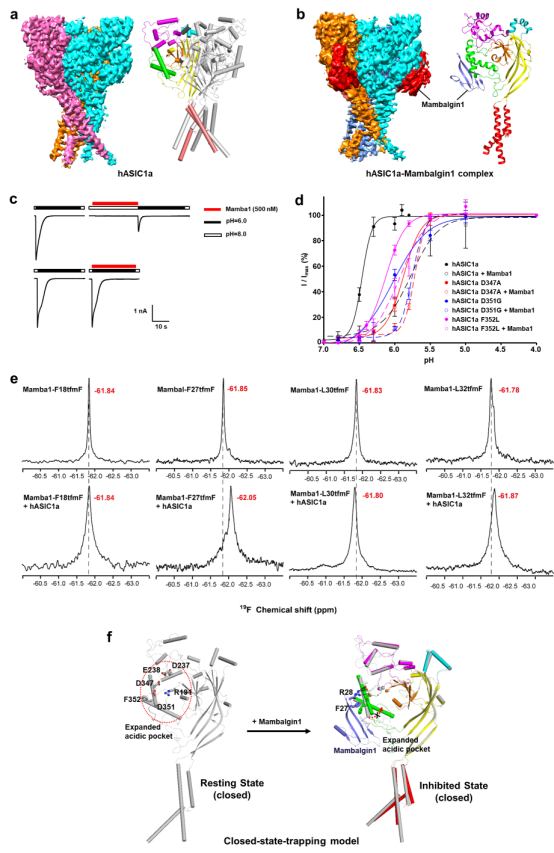
Recently, Tian Changlin of the University of science and technology of China and Liu Lei of Tsinghua University jointly studied the structure of the human acid sensitive ion channel hasic1a and hasic1a and mambalgin1 complex by means of frozen electron microscopy, and comprehensively analyzed them by 19F-NMR and patch clamp electrophysiological function analysis, revealing that the toxin peptide binds and inhibits the hasic1a ion The structure mechanism of the channel.
Hasic1a / mambalgin1 complex structure indicates that the toxin peptide is formed outside the extracellular domain of hasic1a protein through electrostatic and hydrophobic interactions. This fact corrects the previous hypothesis about the interaction between toxin and ASIC channel. Further studies have shown that mambalgin1 selectively binds to the closed ASIC channel, locking the ion channel to the closed configuration. Even if the proton concentration rises, the channel is difficult to open. Therefore, we propose that mambalgin1 toxin can inhibit ASIC channel activity through the closed state rapping mechanism. This new mechanism will provide important clues for the development of novel peptide drugs targeting hasic1a.
In addition, although there is a high similarity between hasic1a and casic1 in general, the differences of amino acids at the specific sites of hasic1a and casic1 lead to different inhibitory effects of mambalgin1 on hasic1a and casic1, which reveals the differences in physical and chemical basis of regulatory effects of mambalgin1 toxin peptides on different species.
If the original article is reprinted, please indicate the source( www.gotopbio.com )”


Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
+86 15021993094
Follow Us:




 Pharma Sources Insight July 2025
Pharma Sources Insight July 2025


