
 Six years of qualification
Six years of qualification
 Audited Supplier
Audited Supplier
In This Store
JenKem Technology provides PEGylation services up to pre-clinical stage, and custom synthesis of PEGylated conjugates. PEGylation is a versatile tool to improve the water solubility, biocompatibility, immunogenicity, and the PK/PD profile of drugs. JenKem Technology’s dedicated and experienced PEGylation group offers two service models to meet your unique PEGylation needs for proteins, peptides, oligonucleotides, and small molecules.

JenKem Technology has patented many PEG-related technologies and respects the intellectual property of others. As of August 2021, JenKem Technology had 86 patent approvals, 43 pending patent applications, several DMFs filed with the FDA, and many other innovative technologies under development.
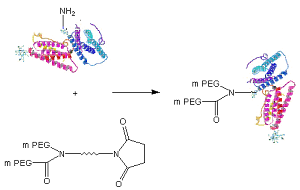
Schematic of protein PEGylation with JenKem® patented Y-Shape PEG NHS
PEGylation Service Model 1
Custom synthesis of PEGylated conjugates, PEG-proteins, PEG-peptides, PEG-polypeptides, PEG-oligonucleotides, PEG-small molecules, and more, using JenKem® catalog PEGs or custom PEG products. JenKem Technology will PEGylate your own protein, peptide, oligonucleotide, or small molecule, and deliver your PEGylated product with a certificate of analysis as a regular end-product, for further testing at your site. This is recommended for an initial evaluation of PEGylation approaches.
PEGylation Service Model 2
Complete PEGylation method development service. Under this service model, JenKem Technology will provide your PEGylated sample, as well as the complete method development package, including developed analytical methods for characterization of your PEGylated product, up to pre-clinical stage. The dedicated PEGylation group can help you identify the right high quality PEG derivatives for your project. Processes developed by JenKem Technology are easily scalable and transferable.
Phase 1: JenKem Technology delivers PEGylated compounds to the customer for pharmaceutical analysis and provides a detailed report including PEGylation reaction conditions, separation and purification processes, and analytical methods for the PEGylated compounds.
1.PEG reagent screening
2.Screening of PEGylation reaction conditions
3.PEGylation process development, including purification
4.Analytical method development
5.Synthesis, characterization and delivery of the PEGylated molecule
Phase 2: JenKem Technology coordinates the IND enabling preclinical study data for PEGylated compounds together with partner CROs, to determine the optimal PEGylated compounds for customers’ application.
1.Quality specification study of PEGylated molecules
2.Formulation study of PEGylated molecules
3.Pharmacokinetics and pharmacodynamics (PK/PD) study of PEGylated molecules
4.Toxicology study of PEGylated molecules
5.IND filing with China FDA
For detailed information about our custom PEGylation services, please contact us at tech@jenkemusa.com.
Example PEGylation Projects
IND Approval Received for PEG-irinotecan Developed by JenKem Technology Co., Ltd. – China FDA approved 3SBio Inc.’s Investigational New Drug (IND) appplication for PEG-irinotecan. 3SBio Inc., a leading biotechnology company in China entered into an exclusive license agreement with JenKem Technology Co., Ltd. for the development, manufacturing and marketing in Mainland China of PEG-irinotecan. 3SBio Inc. intends to develop PEG-irinotecan for metastatic breast cancer and colorectal cancer, and platinum-resistant ovarian cancer and will undergo clinical trials in China. Read More
Out-Licensing Opportunities
We are pleased to offer several other innovative PEG and PEGylation-related technologies for out-licensing as sole and non-exclusive licenses. Please click on the link below for details.
Link to Out-Licensing Opportunities – JenKem Technology USA (jenkemusa.com)

Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
Follow Us:




 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025


