
 Audited Supplier
Audited Supplier
In This Store
Animal vaccines belong to the category of veterinary biological products, which are an important part of biopharmaceuticals.Due to the shortcomings of traditional vaccines and the development of science and technology, the research of many new vaccines, including recombinant vaccines, has become the focus.
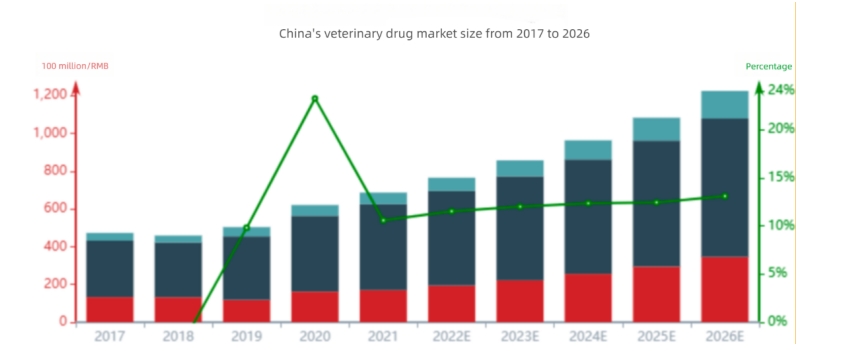
The scale of the animal health industry
I. Genetic Engineered Vaccines
Genetic engineered vaccines are vaccines made by cloning and expressing protective antigen genes using recombinant DNA technology, with the expressed antigen products or the recombinants themselves as the components. They mainly include genetic engineered subunit vaccines, genetic engineered vector vaccines, and genetically deleted live vaccines.
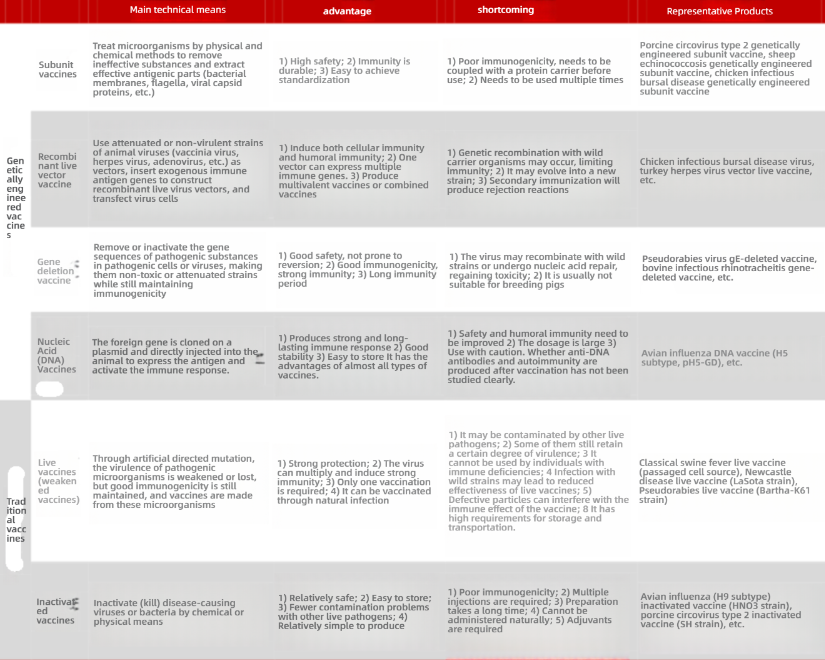
Differences between genetic engineered vaccines and traditional vaccines
In recent years, China's veterinary biological products industry has also made significant progress, with genetically engineered vaccines for porcine circovirus, avian influenza, pseudorabies, swine fever, etc. having been successively launched, and the research and development of bivalent three-component subunit vaccines for foot-and-mouth disease is also steadily progressing. After the upgrading of Chinese vaccines to genetic engineering, the quality gap between them and imported vaccines has been significantly narrowed. BioLink can provide you with complete and diverse solutions to support the research and development of animal health genetic vaccines.
II. Virus-like Particle-based Vaccines
Virus-like particles (VLPs) are large particles assembled from one or several structural proteins of multiple viruses. They do not contain viral nucleic acids and cannot replicate autonomously, but have an overall structure similar to that of viral particles. VLPs have the advantages of high safety and can stimulate an effective immune response through the same pathway as viral particles.
The key to producing biological drugs based on VLPs lies in the ability to prepare high-quality VLPs on a large scale. The preparation of VLPs mainly includes several steps such as cloning and expression of viral structural genes, selection of host expression systems, purification, and identification.
The production of VLPs has certain commonalities, but due to the structural and compositional differences in the construction of different VLPs, there will also be individual issues in the expression, purification, and identification methods of each VLP. Although research on VLPs is increasing and the level is continuously improving, the production process still faces many challenges, including expression levels, assemblies, high yields and purity in purification processes, etc. Therefore, in order to obtain high-quality VLPs products and accelerate the speed of research and development and industrialization, comprehensive optimization is required from upstream construction methods and the selection of expression systems to purification processes and VLPs quality identification methods.
BioLink VLPs Solution:
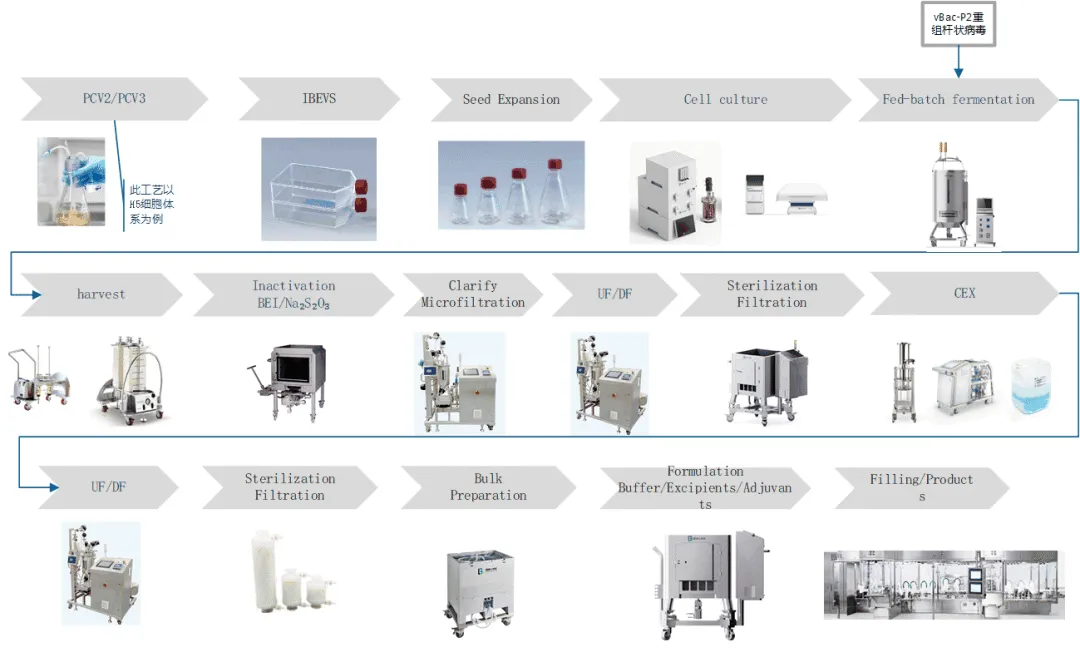
BioLink swine fever subunit vaccine solution
Case share: For porcine circovirus subunit vaccine, a one-step purification method was developed, using MaXtar® SP HR to purify porcine circovirus Cap protein in one step.
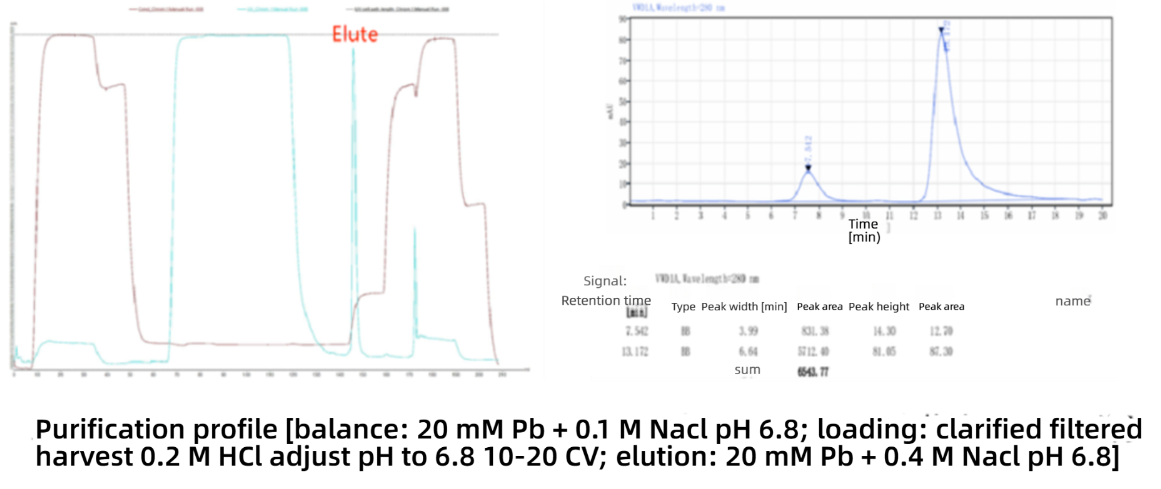
The purity reached 87.30% through HPLC-SEC detection. Repeated experiments and further amplification led to highly reproducible results, successfully achieving the development and amplification of the one-step process for porcine circovirus Cap protein.
Summary: Advantages of MaXtar® SP HR process: high yield, good process reproducibility, simple and operable conditions, and easy to scale up.
III. Nucleic Acid Vaccines
Nucleic acid vaccines mainly include DNA vaccines and mRNA vaccines. Both of them inject the gene sequences encoding pathogen antigens into animals, so that the host cells can translate the corresponding antigen proteins, thereby activating the immune system to produce immune responses.
Currently, in the field of animal health protection, significant potential has been demonstrated in recent years in the research of avian influenza, rabies, African swine fever, and tuberculosis. On November 11, 2023, MSD Animal Health announced that the Sequivity IAV-S NA (swine influenza vaccine, N1 and N2, RNA particles) vaccine has now become a commercial part of its swine product portfolio. The company received a license approval for this vaccine from the United States Department of Agriculture (USDA) in 2022. As a revolutionary swine vaccine platform, SEQUIVITY utilizes RNA particle technology to create customized, prescription-based vaccines against swine influenza A virus strains, porcine circovirus (PCV), rotavirus, and more.
Sequivity IAV-S NA swine influenza vaccine
BioLink Nucleic Acid Vaccine Solutions:
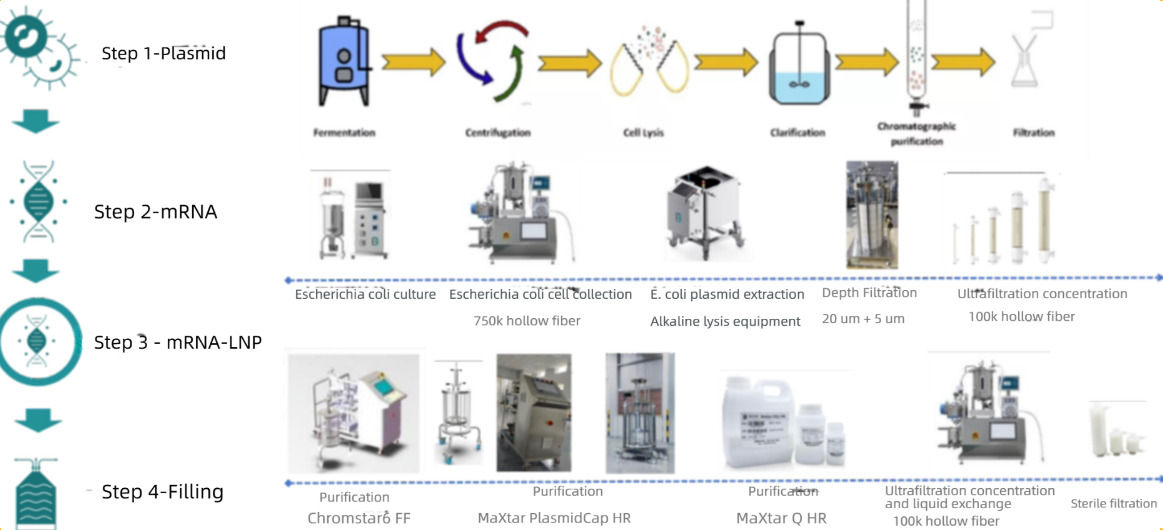
BioLink Nucleic Acid Vaccine Solutions
Process Support:
On the whole, with the development and progress of new vaccines and the application of new technologies, the development and upgrading of product processes can be facilitated, including: high-throughput screening technology can be used to quickly screen out target biomolecules; new technologies such as gene recombination and nucleic acid encoding can be used to produce biological products such as recombinant proteins and vaccines; the automation and information management of the production process have improved production efficiency and quality control.
Support from BioLink:
The above product categories and process schemes are only a part of veterinary vaccines. In practical applications, different combinations and strategies may be adopted according to different diseases and animal species. The promotion of multivalent vaccines and genetically engineered vaccines will further expand the market size of the animal vaccine industry. Multivalent vaccines and new vaccines will become the core products of enterprises in the future.
BioLink can provide customized solution development, including carrier selection, expression system, and optimization and improvement of production processes. With its professional team and rich experience, BioLink can provide comprehensive technical support and solutions to veterinary vaccine enterprises, helping them achieve technological innovation and continuous upgrading, thereby improving product quality, reducing production costs, and enhancing market competitiveness.

Contact Us
Tel: (+86) 400 610 1188
WhatsApp/Telegram/Wechat: +86 13621645194
Follow Us:




 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025


