
How to join PharmaSources as a freelance writer?
The Active Pharmaceutical Ingredient (API) market in China has seen significant growth and development in recent years. China has emerged as a major player in the global pharmaceutical industry, with a well-established API manufacturing sector.
APIs are the key components in pharmaceutical formulations that have therapeutic effects. China has become one of the leading suppliers of APIs globally, owing to factors such as lower manufacturing costs, a large pool of skilled labor, and a well-developed chemical industry.
Top API companies in China
China is home to several leading API companies that play a significant role in the global pharmaceutical industry. Here are some of the top API companies in China:
Zhejiang Huahai Pharmaceutical Co., Ltd.: Huahai Pharmaceutical is one of the largest API manufacturers in China. It specializes in the production of cardiovascular and antiviral APIs, such as Losartan and Lamivudine.
Jiangsu Hengrui Medicine Co., Ltd.: Hengrui Medicine is a renowned Chinese pharmaceutical company engaged in the research, development, and manufacturing of APIs and finished dosage forms. It focuses on oncology, anti-infectives, and cardiovascular therapeutic areas.
Northeast Pharmaceutical Group Co., Ltd.: Popularly known as NEPG, Northeast Pharmaceutical Group is a state-owned pharmaceutical company with expertise in manufacturing APIs, intermediates, and finished dosage forms. It produces a wide range of APIs for various therapeutic areas.
Zhejiang Medicine Co., Ltd.: Zhejiang Medicine is a China-based pharmaceutical company that operates in the research, development, production, and sale of APIs and pharmaceutical intermediates. It has a diversified portfolio of APIs, including antipyretic, analgesic, cardiovascular, and anti-infective agents.
Shandong Xinhua Pharmaceutical Co., Ltd.: Xinhua Pharmaceutical is a leading Chinese pharmaceutical company involved in the production of APIs, preparations, and intermediates. It specializes in APIs for cardiovascular, anti-infective, and central nervous system therapeutic areas.
CSPC Pharmaceutical Group Limited: CSPC Pharmaceutical is a major Chinese pharmaceutical company engaged in the manufacturing and sale of APIs and finished pharmaceutical products. It offers a wide range of APIs and has a strong presence in the central nervous system, cardiovascular, and anti-infective segments.
Sichuan Chengkang Pharmaceutical Co., Ltd.: Chengkang Pharmaceutical is a well-known API manufacturer in China with a focus on cephalosporin antibiotics. It has a comprehensive portfolio of APIs and intermediates used in the production of antibiotics.
Luye Pharma Group Ltd: Luye Pharma is a Chinese pharmaceutical company that specializes in the research, development, production, and sale of APIs and pharmaceutical products. It covers various therapeutic areas, including oncology, central nervous system disorders, and respiratory diseases.
Zhejiang Hisun Pharmaceutical Co., Ltd.: Hisun Pharmaceutical is a prominent Chinese pharmaceutical company involved in the production of APIs, formulations, and biopharmaceuticals. It offers APIs for various therapeutic areas, including anti-tumor, anti-infective, cardiovascular, and gastrointestinal drugs.
Zhejiang NHU Co., Ltd.: NHU is a leading manufacturer of APIs, vitamins, and fine chemicals in China. It produces a wide range of APIs, including antibiotics, vitamins, and amino acids.
Please note that this is not an exhaustive list, and there are other notable API companies in China. The ranking and prominence of companies may vary based on factors such as revenue, market share, and specialization.
Top API products in China
China is known for its vast production of Active Pharmaceutical Ingredients (APIs) across various therapeutic areas. Here are some of the top API products that are manufactured in China:
Paracetamol (Acetaminophen): Paracetamol is a widely used analgesic and antipyretic API. It is used to relieve pain and reduce fever. China is a major global supplier of paracetamol API.
Amoxicillin: Amoxicillin is a broad-spectrum antibiotic used to treat various bacterial infections. China is a significant producer of amoxicillin API, catering to both domestic and international markets.
Metformin: Metformin is an oral medication used for the treatment of type 2 diabetes. It helps lower glucose levels in the blood. China is a major manufacturer of metformin API.
Atorvastatin: Atorvastatin is a cholesterol-lowering medication that belongs to the statin class of drugs. It is used to manage high cholesterol levels and reduce the risk of cardiovascular diseases. China is a significant producer of atorvastatin API.
Losartan: Losartan is an angiotensin II receptor blocker (ARB) used to treat hypertension (high blood pressure). China is a major manufacturer and exporter of losartan API.
Ciprofloxacin: Ciprofloxacin is a fluoroquinolone antibiotic used to treat bacterial infections. It is widely produced in China and has gained popularity for its efficacy and broad spectrum of activity.
Levofloxacin: Levofloxacin is another fluoroquinolone antibiotic used to treat various bacterial infections. China is a leading manufacturer of levofloxacin API.
Omeprazole: Omeprazole is a proton pump inhibitor (PPI) used to reduce stomach acid production and treat conditions such as gastroesophageal reflux disease (GERD) and stomach ulcers. China is a major producer of omeprazole API.
Montelukast: Montelukast is a leukotriene receptor antagonist used to manage asthma and allergic rhinitis. China is a significant manufacturer of montelukast API.
Sildenafil Citrate: Sildenafil citrate is the active ingredient in the famous erectile dysfunction medication, Viagra. China produces a significant amount of sildenafil citrate API for both domestic and international markets.
Please note that this list is not exhaustive, and China manufactures a wide range of APIs for various therapeutic areas. The popularity and demand for specific API products may vary over time and across different regions.
API Marketing Application Policies in China
The marketing application policies for Active Pharmaceutical Ingredients (APIs) in China are governed by the National Medical Products Administration (NMPA), which is responsible for regulating pharmaceuticals in the country. Here are some key aspects of the marketing application policies for APIs in China:
Drug Registration: In China, APIs are considered as part of the overall drug registration process. In order to market APIs in China, manufacturers need to obtain a drug registration approval from the NMPA. The registration process involves submission of comprehensive data on the quality, safety, and efficacy of the API.
Good Manufacturing Practices (GMP): API manufacturers are required to comply with China’s GMP regulations. They must establish and maintain a GMP-compliant manufacturing facility, which is subject to inspection by the NMPA. Compliance with GMP standards is essential to ensure the quality and safety of APIs.
Impurity Control: China has specific requirements regarding the control of impurities in APIs. Manufacturers must conduct comprehensive impurity profiling and demonstrate control over potential impurities that may be present in the API. The impurity limits and testing methods must comply with relevant regulations.
Data Requirements: When submitting a marketing application for an API, manufacturers need to provide comprehensive data on the quality, safety, and efficacy of the API. This includes data on the synthesis process, characterization, analytical methods, stability, impurity profile, and any other relevant information.
Bioequivalence Studies: In certain cases, if an API is used as a reference standard for bioequivalence evaluation, the manufacturer may need to conduct bioequivalence studies to demonstrate that the generic version of the API is therapeutically equivalent to the reference drug.
Post-Marketing Surveillance: Once an API is approved for marketing in China, manufacturers are required to comply with post-marketing surveillance requirements. This involves monitoring the safety and efficacy of the API in the real-world setting, and reporting any adverse events or product quality issues to the regulatory authorities.
It is important to note that the exact requirements and procedures may vary depending on the specific API and its intended use. Manufacturers are advised to consult the relevant guidelines and regulations issued by the NMPA and seek professional assistance for the marketing application process in China.





 ALL
ALL Pharma in China
Pharma in China Pharma Experts
Pharma Experts Market News
Market News Products Guide
Products Guide Brand Story
Brand Story

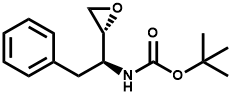
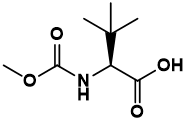


















 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025








