-
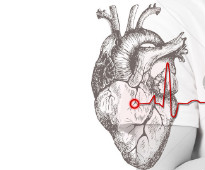
Cardiac monitoring for high-risk breast cancer patients ‘should be prioritised’
europeanpharmaceuticalreview
April 19, 2019
Cardiac monitoring of high-risk breast cancer patients should be prioritised, after research revealed low rates of heart monitoring seen in the patients receiving trastuzumab.
-
AstraZeneca and Daiichi Sankyo sign $6.9bn cancer drug development deal
pharmaceutical-technology
April 11, 2019
AstraZeneca has entered a deal worth up to $6.9bn with Daiichi Sankyo to develop and commercialise cancer drug trastuzumab deruxtecan...
-
Phase III KATHERINE trial crossed early reporting boundary and met its primary endpoint
europeanpharmaceuticalreview
December 06, 2018
A phase III clinical trial showed that neoadjuvant chemotherapy and trastuzumab reduced the risk of recurrence of HER2-positive early-stage breast cancer…
-
Trastuzumab taken with carvedilol leads to less heart damage
europeanpharmaceuticalreview
December 06, 2018
A team of Iranian researchers have determined that trastuzumab taken with a heart drug leads to less heart damage in breast cancer patients…
-
Roche's Kadcyla beats Herceptin breast cancer disease recurrence and death risk
pharmafile
October 19, 2018
Roche has unveiled new data for its antibody-drug conjugate Kadcyla (trastuzumab emtansine) in the treatment of patients with HER2-positive early breast cancer (eBC) who...
-
Roche’s Kadcyla shows promise in early breast cancer
pharmatimes
October 19, 2018
Roche has announced that its antibody drug conjugate Kadcyla cut the risk of disease recurrence when given after neoadjuvant treatment and surgery to certain breast cancer patients with residual disease.
-
Australia gives its nod to Trastuzumab Biosimilar
biospectrumasia
August 10, 2018
The trastuzumab biosimilar will join 2 other anticancer biosimilars on the list of TAG’s approved biosimilars.
-
Napp secures distribution rights to Celltrion’s Herzuma
pharmatimes
July 23, 2018
Napp Pharmaceuticals has secured itself exclusive UK distribution rights to Celltrion Healthcare’s Herceptin biosimilar Herzuma.
-
US delay for Celltrion's Rituxan, Herceptin biosimilars
pharmatimes
July 18, 2018
The US Food and Drug Administration has rejected Celltrion’s submissions for biosimilars to Roche drugs Rituxan and Herceptin.
-
U.S. FDA accepts Biologics License Application (BLA) for Mylan and Biocon’s proposed Biosimilar Tras
pharmaasia
February 07, 2017
If approved, MYL-1401O has potential to be the first Biosimilar Trastuzumab in the U.S.

 ALL
ALL Pharma in China
Pharma in China Pharma Experts
Pharma Experts Market News
Market News Products Guide
Products Guide Brand Story
Brand Story






 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025








