Hydroxypropyl Betadex Oral pharma grade USP Standard
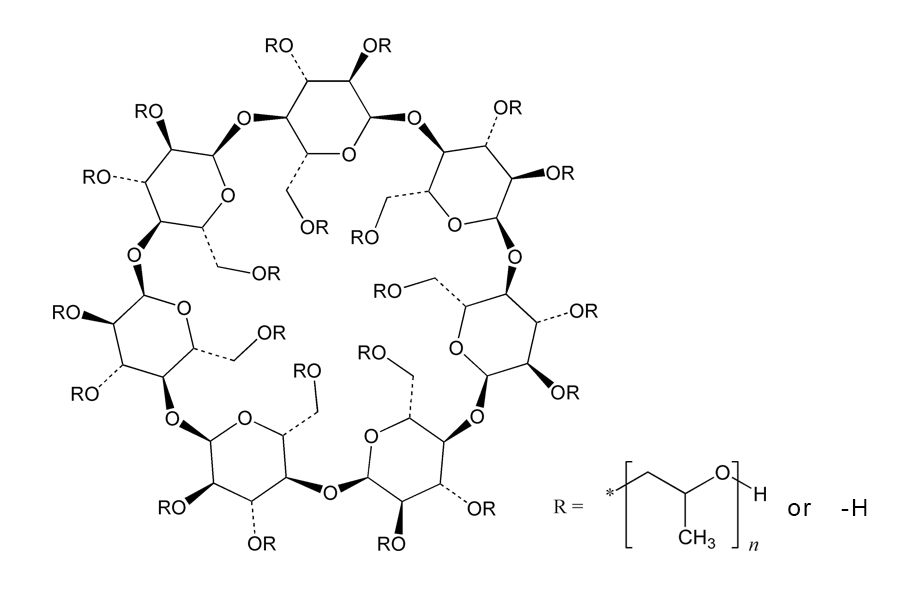
Product name: Hydroxypropyl Betadex
CAS No.:128446-35-5
DMF No.:030168
Standard :CP / USP /EP
Grade: Oral grade / Pharma grade
Product Name: Hydroxypropyl Betadex oral grade
......................................................................................................
Synonyms: Hydroxypropyl-β-Cyclodextrin; Hydroxypropyl beta Cyclodextrin
......................................................................................................
Molecular Formula: C42H70-nO35(C3H8O2)n
......................................................................................................
Molecular Weight: 1134.98+58n
|





 Pharma Sources Insight January 2025
Pharma Sources Insight January 2025


